An experimental antibiotic is being developed that is able to neutralize a wide range of drug-resistant, gram-positive bacteria – pathogens that protect themselves in a slimy protective shield called a biofilm, designed by nature to keep threats at bay.
Bacterial infections are extremely difficult to treat when pathogens are protected by a biofilm. The film forms as a result of bacterial colonies growing together in a tough and protective matrix. Infections from bacteria protected by biofilms are often chronic and span a complex spectrum: Tooth infections that lead to tooth loss can be exacerbated by biofilms. Fatal drug-resistant lung infections, bacterial infiltration of the pericardium, wound infections, and even blood infections can all be complicated by the presence of biofilms.
Treating biofilm-shielded bacteria with antibiotics is challenging, doctors say, because many conventional antibiotics can’t penetrate the mucus to kill the active bacteria.
In Australia, Dr. Mark Blaskovich of the Center for Superbug Solutions at the University of Queensland’s Institute for Molecular Bioscience worked with an international team to tackle resistant infections involving biofilms. Blaskovich and colleagues name their experimental drug MCC5194 and describe it as a modified version of vancomycin, the potent antibiotic supported by decades of use.
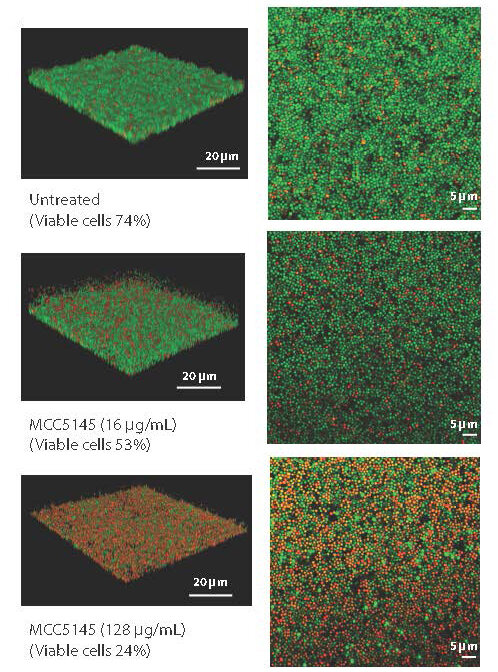
The difference between MCC194 and unmodified vancomycin is that the experimental drug acts like a torpedo when hitting biofilms. In pre-clinical testing, MCC5194 killed key gram-positive bacterial threats such as methicillin-resistant Staphylococcus aureus – MRSA – and disrupted bacterial biofilm. The drug was also effective in tests against other gram-positive bacteria and eliminated their biofilms as well.
For patients worldwide, the annual morbidity and mortality from biofilm-forming infectious bacteria is enormous. Besides MRSA, another difficult-to-treat biofilm-forming bacterium is Streptococcus pneumoniae. But as the global health crisis of antimicrobial resistance has grown inexorably, solutions have been few. Worse, the current research pipeline is not producing enough new antibiotics to solve the problem, which Blaskovich and colleagues attribute at least in part to economic factors. The development of antibiotics is not a high priority for pharmaceutical companies.
“Drug-resistant infections with Gram-positive bacteria still represent a significant burden on the public health system, with two bacteria, Staphylococcus aureus and Streptococcus pneumoniae, responsible for over 1.5 million drug-resistant infections in the United States alone,” Blaskovich wrote Science Translational Medicine.
In 2019, 250,000 deaths were attributed to these pathogens worldwide, Blaskovich added, noting that “drug-resistant gram-positive bacterial infections remain a critical problem and new treatments against them are needed in the clinic.”
The research, which involved teams from the United States, Switzerland and the United Kingdom, exposed hundreds of clinical isolates of MRSA and other gram-positive bacteria to MCC194. In addition to testing infected human cells, the team also tested the drug in a murine animal model. A series of experiments involved thigh infection in the mice. Another part of the tests evaluated the efficacy of MCC194 against septicemia caused by Streptococcus pneumoniae.
The new compound outperformed the approved antibiotic vancomycin, as shown by the results reported in Science Translational Medicineand destroyed hard-to-remove bacterial biofilms while resulting in a low rate of resistance.
The team said their findings suggest that MCC194 could one day be part of the medical arsenal targeting bacterial infections that have become increasingly resistant to standard antibiotic therapy. The hope is that MCC194 will become a drug exclusively for drug-resistant Gram-positive bacteria, particularly those that are biofilm-infested.
Gram-positive bacteria are those that absorb the dye known as crystal violet staining, which gives these pathogens a deep purple hue when viewed under a light microscope. Gram-positive bacteria have a thick peptidoglycan outer layer that retains the stain.
In contrast, gram-negative bacteria do not readily absorb the crystal violet stain. They have a relatively thin layer of peptidoglycan sandwiched between an inner and outer cell membrane. Gram-negative bacteria include some of the most notorious drug-resistant species: Pseudomonas aeruginosa, Klebsiella, Acinetobacter, and E. coli.
These pathogens often appear pink when viewed through a light microscope after crystal violet staining. Gram-negative species, like their gram-positive counterparts, are readily capable of forming biofilms. P. aeruginosa, a threat of fatal lung infections, is characterized by a persistent biofilm.
Although efforts to combat biofilm-forming bacteria are not as robust as they should be, most ongoing research is largely focused on gram-negative species and the complex nature of their cell membranes, Blaskovich and colleagues say.
This focus, they claim, overlooks an unavoidable need for drugs to treat patients infected with persistently resistant strains of Gram-positive bacteria. “We have developed a preclinical glycopeptide antibiotic that has excellent potency against hundreds of isolates of methicillin-resistant S. aureus and other gram-positive bacteria,” explained Blaskovich, adding “the optimized compound.” [MCC194] was more potent than its parent vancomycin and showed a low rate of resistance, suggesting that this candidate compound may warrant further development.”
New drug candidate fights more than 300 drug-resistant bacteria
Mark AT Blaskovich et al, A lipoglycopeptide antibiotic for gram-positive biofilm-borne infections, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abj2381
© 2022 Science X Network
Citation: Experimental Antibiotic Torpedoes the Protective Slime That Makes Resistant Bacteria Harder to Fight (2022 October 13) Retrieved October 13, 2022 from https://medicalxpress.com/news/2022-10-experimental-antibiotic-torpedoes- slime-resistant.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#Experimental #antibiotic #torpedoes #protective #mucus #resistant #bacteria #harder #fight

Leave a Comment