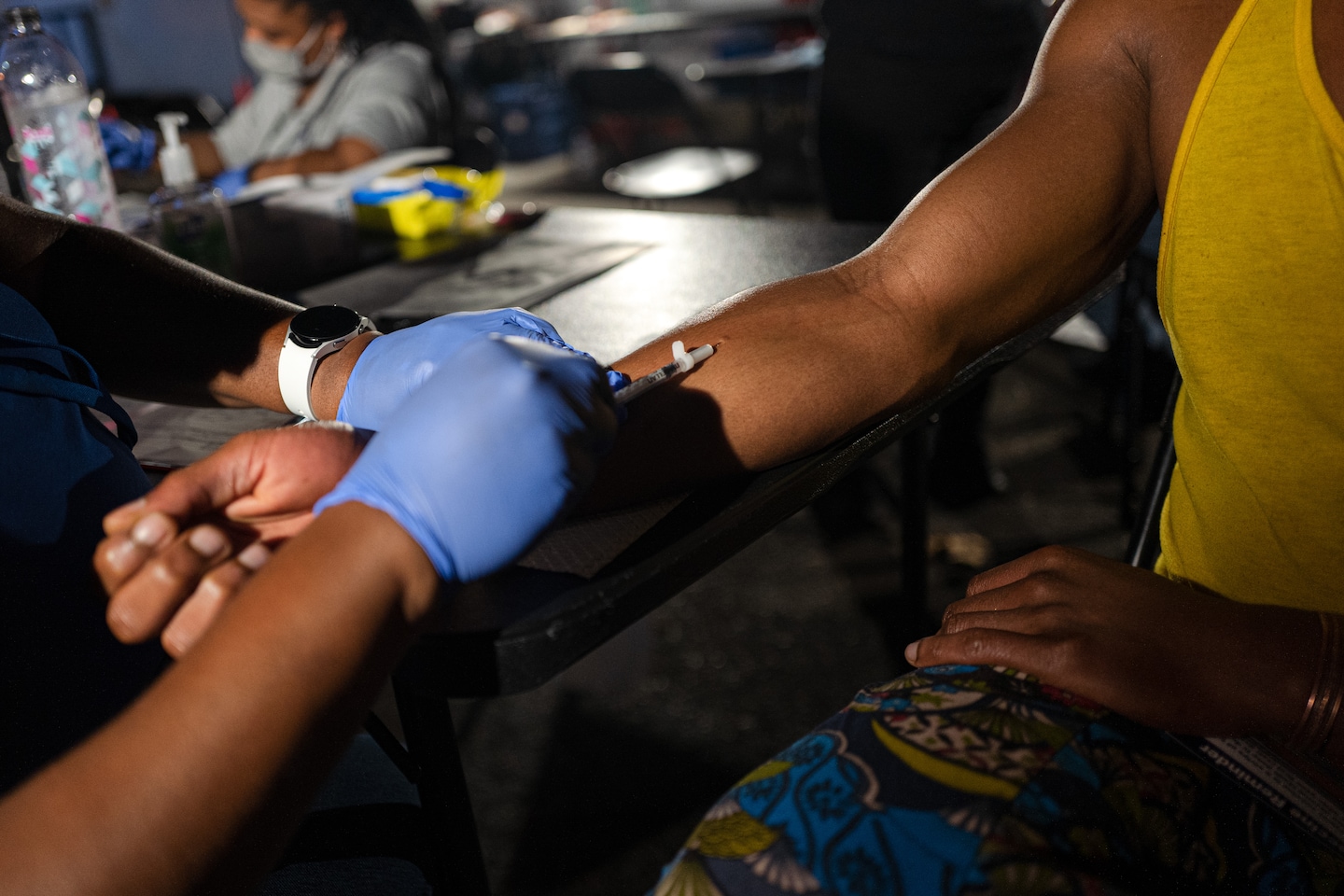
As a result, the FDA announced in August that it will issue an emergency use authorization for the Jynneos monkeypox vaccine to be administered by intradermal injection — where the vaccine is delivered into the immune cells between the layers of skin, often in the forearm, rather than deep injection in fat or muscle tissue in the shoulder. While the method has been used for other vaccines, it had not previously been approved by the United States for the monkeypox vaccine. A smaller amount of vaccine is used, meaning one dose can be divided between five people.
Two months later, the outbreak appears to be waning, and many parts of the country are seeing monkeypox vaccine appointments more frequently than earlier in the summer, when getting a vaccine was like “winning the sweepstakes or getting concert tickets,” one recipient said.
However, the provisional vaccination schedule was not without its downsides. Intradermal injection can leave a painful, itchy red spot for weeks, potentially increasing the stigma of an outbreak that primarily affects gay men, and long-term discoloration or scarring can occur. The FDA’s emergency use approval of the intradermal method was largely based on a single 2015 study that showed that intradermal and traditional “subcutaneous” injections of the vaccine elicited similar immune responses.
All of this has led to lingering concerns about stigma, discomfort, and effectiveness for some people.
Cooper Newnam, a 25-year-old Phoenix resident, said he skipped the second injection in the two-dose regimen because of concerns about the intradermal injection method. Other gay men he knew who had their second doses in their forearms were left with a red welt, a visual reminder of the outbreak that altered her feelings at work and “she felt kind of marginalized.” , he said.
“I felt like nobody told us, ‘You’re about to have a clear sign of monkeypox on your body, even though you get vaccinated.’ Still, to some degree, it’s a clear sign because the straight communities aren’t going to have that huge bump on their arm,” Newnam said. “It was another exclusion.”
Max, a 36-year-old living in New York City, said he wasn’t concerned about the safety of the intradermal injection, but whether it would be as effective as his first dose, which was given via the traditional method.
“I was concerned that what I was receiving — a fifth the dose in this other way — might not be as effective as the recommended dose given to me in my arm the first time,” said Max, who reports on the condition said his full name will not be used due to concerns about professional implications and the stigma attached to monkeypox.
The pain is “not terrible” but enough to wake him up in the middle of the night, he said. He wondered, “What did I do to myself to get this?”
The intradermal injection method is “a more difficult technique to learn,” said William Schaffner, an infectious disease specialist at Vanderbilt University Medical Center. “You have to use a smaller needle, and you have to be very skilled at learning that because you don’t want to go through the skin.” Too deep, and the smaller dose won’t be enough to elicit an adequate immune response, he said.
Senior federal health officials, including Rochelle Walensky, director of the Centers for Disease Control and Prevention, wrote in an opinion piece published in the New England Journal of Medicine last week that “despite limited clinical evidence” data suggested the intradermal syringes were producing an equitable immune response “to prevent monkeypox infection and disease”.
The CDC and FDA are “committed to conducting the studies necessary to ensure that our expectations are met,” the article said. Meanwhile, people at high risk of infection are advised to receive both doses and vaccine manufacturers are urged to test the method to “broaden our understanding of it”.
But even the maker of the vaccine used in the US has expressed concerns about the intradermal method to the Biden administration. “We have some reservations…due to the very limited safety data available,” wrote Paul Chaplin, chief executive officer of Bavaria Nordic, in a letter sent to senior administration officials and obtained by the Washington Post in August. Chaplin said “it would have been wise” to do more studies before changing vaccination strategy.
In its response, the FDA told Chaplin that the continued spread of the virus “necessitated that the FDA vigorously review all available vaccine options to provide protection to the population at risk.”
When the FDA began looking at the intradermal method, it determined it would be an “effective strategy” with “an acceptable safety profile,” a senior US health official said in an interview, speaking on condition of the Anonymity to speak openly about the administration’s response to the outbreak.
“It was clear that there were a few compromises,” the official said, noting the discomfort and duration of the injection site markings. Despite these drawbacks, “being able to actually make the vaccine available to everyone” who was at risk of infection “was really an attractive option for us,” the official said, noting that the quality of the 2015 study was “really good.”
Schaffner agreed that the study was “very well done” and that there was a long history of other vaccines being given intradermally. When the FDA announced its emergency use approval, “it came as a bit of a surprise to all of us,” he said, although the data on its effectiveness is “very reassuring.”
Despite concerns, Schaffner said it was “absolutely” better to give the vaccines intradermally than not at all.
Joe Wood, 34, said he still needs his second dose but has had no problems with the intradermal injection. “I trust what the CDC and the FDA say,” he said. “Protection would be my number one priority, so personally I’m not at all worried about that happening.”
The CDC advises people under the age of 18 and people with a history of keloid scars — thick, raised scars — to get the vaccine through the “standard regimen.” Concerns about discoloration or scarring are “real — we can’t trivialize it,” Schaffner said.
He wondered if there could be procedures to remove what was left of the welts in a similar way to removing tattoos, “because it would make a lot of people happier if they didn’t walk around with that mark of Cain on their forearm. I think that’s a real problem.”
Joey Uy, 25, received a second dose intradermally Monday in New York City. “This one is definitely different than the first one,” which was administered in the shoulder, said Uy, who wore long sleeves to avoid scratching the injection site and bathed frequently to keep it clean.
“It’s similar to a mosquito bite, but three or four times worse,” Uy said. “I try not to think about it.”
#Monkeypox #cases #concerns #intradermal #vaccine #remain

Leave a Comment