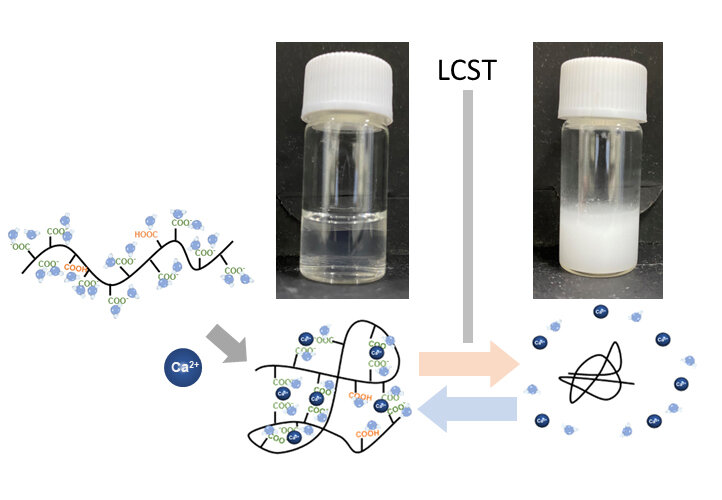
The novel thermoresponsive polymer was prepared by adding ions to polymers and aqueous solutions. Its thermoresponsiveness can be easily controlled by changing the kind and the mixing ratio of the ionic species. Photo credit: Harada, OMU
Temperature-sensitive or thermoresponsive polymers, often referred to as smart materials, are gaining attention due to their ability to respond to external temperature changes, enabling a wide range of applications. To make this smart material even smarter by improving the flexibility of its temperature response, scientists at Osaka Metropolitan University have developed a novel polymer whose temperature response can be easily adjusted by changing the kind and mixing ratio of ionic species. Their results were published in macromolecules.
Polymers that exhibit temperature-related changes in their physicochemical properties are referred to as thermoresponsive polymers. They are of two types: lower critical solution temperature (LCST) polymers and upper critical solution temperature (UCST) polymers. Above a certain temperature, the former are insoluble while the latter are soluble.
In LCST-type thermoresponsive polymers, with increasing temperature, the polymer–solvent interaction decreases and the polymer–polymer interaction becomes dominant, leading to the precipitation of polymers from the solvent. Conversely, in UCST-type thermoresponsive polymers, with increasing temperature, the polymer-polymer interaction decreases and the polymer-solvent interaction becomes dominant, leading to dissolution. This indicates that the affinity between the polymer and the solvent is an important factor in most thermoresponsive polymers.
Traditionally, polymer–solvent interaction is used to regulate thermoreactivity in the design of thermoresponsive polymers. However, attention has recently focused on a new technique that regulates thermoreactivity by adding a third component. This technique often uses organic solvents, but in order to develop materials such as those for drug delivery systems, it is necessary that water, which is harmless to the human body, be used as a solvent.
The research team, led by Professor Atsushi Harada of Osaka Metropolitan University’s Graduate School of Engineering, used water as a solvent and developed a thermoresponsive LCST-type polymer by adding alkaline earth metal ions – which are divalent cations – to polymers and aqueous solutions. They managed to regulate the thermoresponsive properties simply by changing the type and mixing ratio of ions. This differs from the conventional technique, which can only regulate the thermoreactivity by changing the structure of the polymers.
‘We have developed a novel polymer that responds to temperature in the presence of certain ions,’ Professor Harada concluded. “We anticipate that it will be used as an analytical reagent for metal ion sensing devices and as a material for drug delivery systems.”
Self-templating, solvent-free supramolecular polymer synthesis
Junya Emoto et al, Thermoresponsiveness of Carboxylated Polyallylamines Induced by Divalent Counterions as Ionic Effectors, macromolecules (2022). DOI: 10.1021/acs.macromol.2c00795
Provided by Osaka Metropolitan University
Citation: Smart Materials: Metal Cations Regulate Thermoresponsive Polymers (2022 October 14) Retrieved October 14, 2022 from https://phys.org/news/2022-10-smart-materials-metal-cations-thermoresponsive.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#Smart #materials #Metal #cations #regulate #thermoresponsive #polymers

Leave a Comment