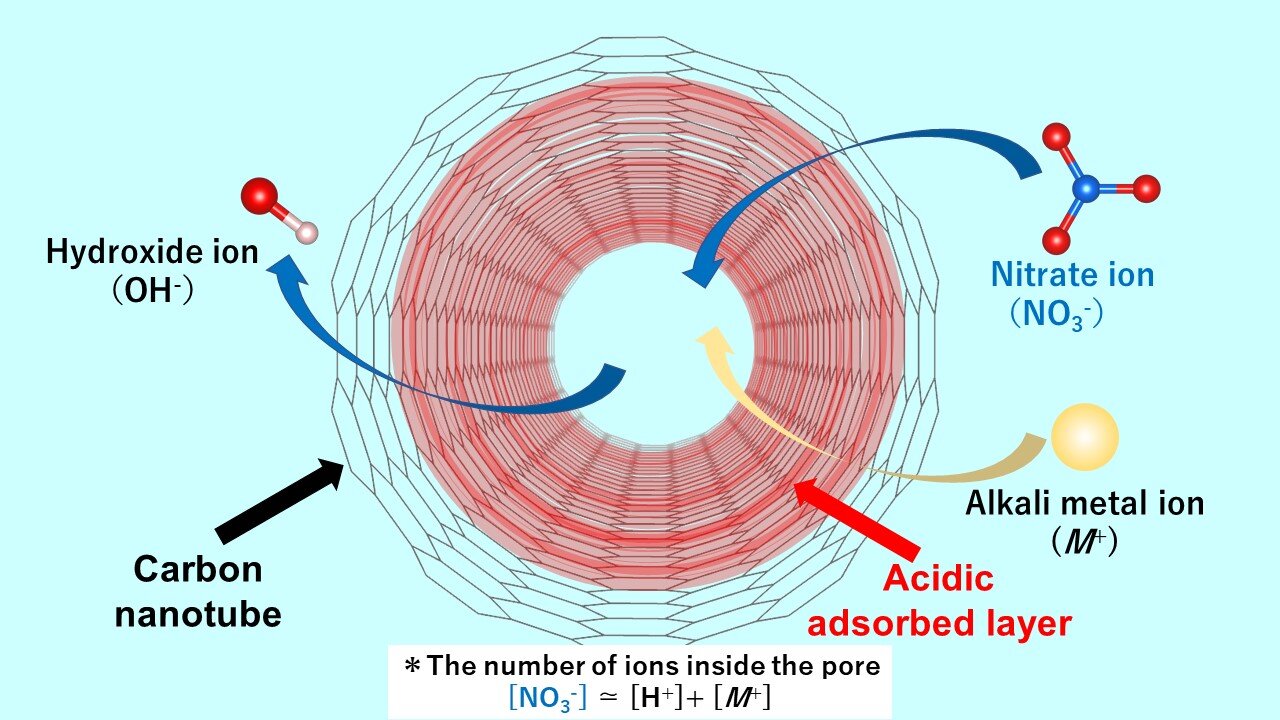
The acid-adsorbed layer enhances the nano-confinement of nitrate anion impurities in single-walled carbon nanotubes (SWCNT) due to the strong confinement by the pore and the strong interaction between the layer and the anion. When the nitrate ions are adsorbed, the hydroxide ions are desorbed from the nanospace. Thus, the aqueous solution exhibits alkaline properties. Credit: Takahiro Ohkubo from the Okayama University Department of Chemistry, Graduate School of Natural Science and Technology, Okayama University
Efficient purification processes that separate contaminants from air and water are necessary to sustain life on earth. To this end, carbon materials have long been used to deodorize, separate and remove harmful anion impurities by adsorption. Until now, the detailed mechanism by which carbon purifies water has remained a mystery. In addition, it is not known whether the aqueous solution adsorbed on the carbon material is acidic, alkaline or neutral.
To fill these gaps, researchers led by Dr. Takahiro Ohkubo, Associate Professor at the Department of Chemistry, Faculty of Natural Science and Technology, Okayama University, Japan, the basic mechanism by which anions are adsorbed by carbon nanopores.
In a recent article made available online and published in September 16, 2022 Journal of Colloid and Interface Sciencesthe researchers report the use of Raman spectroscopic tools to study the adsorption of nitrate ions through the cylindrical pore of single-walled carbon nanotubes (SWCNT).
dr Ohkubo and his colleagues succeeded in deciphering the mechanism of acid layer formation near the pore walls. It turns out that when an aqueous solution containing ions penetrates into the carbon material, even if the aqueous solution is neutral, an acidic aqueous layer containing protons is formed, which maintains a stable state. dr Commenting on the novelty and fundamental nature of their work, Ohkubo said: “Until now, there have been no reports proving the existence of acidic adsorption layers formed in nanotubes made of carbon materials.”
The research team, which also includes Dr. Nobuyuki Takeyasu, associate professor at the same faculty of Okayama University, found that the acidic layer facilitates efficient adsorption of the negatively charged nitrate anion impurities, with the adsorbed amount of nitrate ions being much larger than the cations or the positively charged groups. In addition, hydroxide ions are generated as counter ions. The anions present in the bulk solution are exchanged with the hydroxide ions in the SWCNT, making the aqueous solution alkaline.
The team studied anion adsorption using several alkali metal nitrates, including lithium nitrate, sodium nitrate, rubidium nitrate and cesium nitrate solutions. They found that more nitrate ions are adsorbed than metal ions. The amount of proton adsorption was almost the same regardless of the type of alkali metal ion used. dr Ohkubo says, “The acidic layer in the pore can strongly adsorb the nitrate anion species, both due to the strong confinement by the pore and the strong interaction between the layer and the anion.”
Indeed, the findings are important steps towards the design and development of carbon nanotubes suitable for ion adsorption and purification of water and air. The purification mechanism presented in this research is a novel model that explains the alkalinity of the aqueous dissolution medium, which until now has been a mystery. The researchers note that the results of their study strongly suggest the need to neutralize water before use when ionic impurities are trapped by carbon materials.
Another notable contribution of this study is the demonstration that the interface of nanomaterials is a novel chemical reaction field that could guide further experiments. Taken together, this work brings our understanding of the mechanism of anion adsorption by carbon to the next level and paves the way for novel carbon nanotubes as efficient purifiers.
The subtle role of surfaces in ionic stickiness
Takahiro Ohkubo et al, Acid layer enhanced nano-encapsulation of anions in cylindrical pores of single-walled carbon nanotubes, Journal of Colloid and Interface Sciences (2022). DOI: 10.1016/j.jcis.2022.09.070
Provided by Okayama University
Citation: Acidic layer in single-walled carbon nanotubes facilitates containment of anion impurities (2022 October 20), retrieved October 20, 2022 from https://phys.org/news/2022-10-acidic-layer-single-walled-carbon-nanotubes .html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#acidic #layer #singlewalled #carbon #nanotubes #facilitates #entrapment #anion #impurities

Leave a Comment