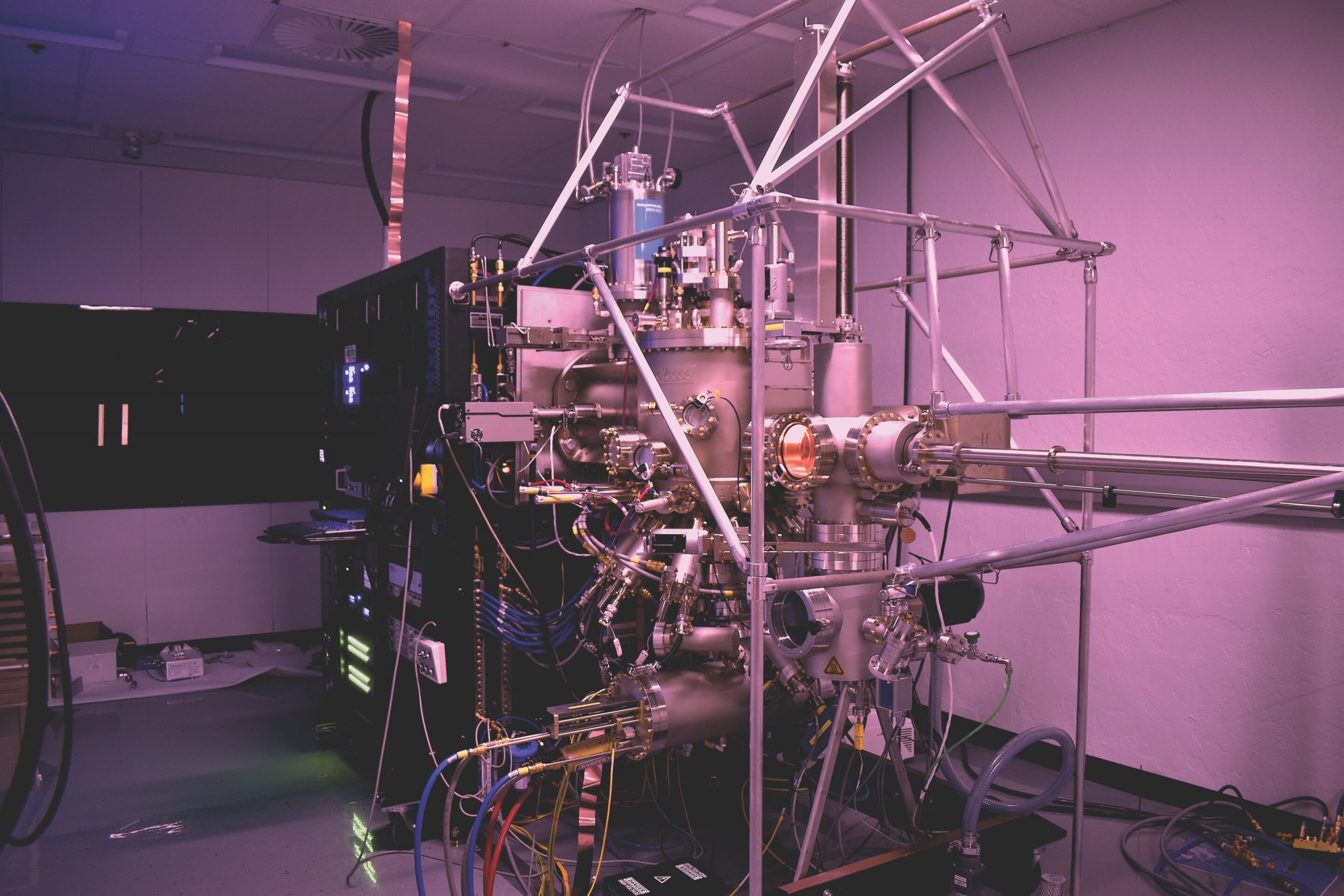
INL researchers have built a lab around molecular beam epitaxy (MBE), a process that produces ultrathin layers of material with a high degree of purity and control. Photo credit: Idaho National Laboratory
Packed with tunnel particles, electron wells, enchanted quarks, and zombie cats, quantum mechanics takes everything Sir Isaac Newton taught about physics and throws it out of hand.
Every day researchers uncover new details about the laws that govern the smallest building blocks of the universe. These details not only advance the scientific understanding of quantum physics, but also hold the potential to unlock a variety of technologies, from quantum computers to lasers to next-generation solar cells.
But there is one area that remains a mystery even in this most mysterious of sciences: the quantum mechanics of nuclear fuel.
Exploring the frontiers of quantum mechanics
To date, most basic scientific research into quantum mechanics has focused on elements such as silicon because these materials are relatively inexpensive, easy to obtain, and easy to process.
Now researchers at the Idaho National Laboratory plan to explore the frontiers of quantum mechanics with a new synthesis lab capable of working with radioactive elements like uranium and thorium.
An announcement about the new laboratory appears online in the journal nature communication.
Uranium and thorium, which are part of a larger group of elements called actinides, are used as fuels in nuclear power plants because they can undergo nuclear fission under certain conditions.
However, the unique properties of these elements, particularly the arrangement of their electrons, also mean that they could exhibit interesting quantum mechanical properties. In particular, the behavior of particles in special, extremely thin actinide materials could advance our understanding of phenomena such as quantum wells and quantum tunneling.
To study these properties, a team of researchers set up a lab around molecular beam epitaxy (MBE), a process that produces ultra-thin layers of material with a high degree of purity and control.
“The MBE technique itself is not new,” said Krzysztof Gofryk, scientist at INL. “It’s widespread. What’s new is that we’re applying this method to actinide materials – uranium and thorium. At the moment, this ability doesn’t exist anywhere else in the world that we know of.”
The INL team conducts basic research – science for the sake of knowledge – but the practical application of these materials could lead to some important technological breakthroughs.
“At this point, we’re not interested in building a new qubit [the basis of quantum computing]but we’re thinking about what materials could be useful for that,” Gofryk said. “Some of these materials could be interesting for new memory banks and spin-based transistors, for example.”
Both memory banks and transistors are important components of computers.
molecular beam epitaxy
To understand how researchers make these very thin materials, imagine an empty pit of balls in a fast food restaurant. Blue and red balls are thrown into the pit one at a time until they form a single layer on the floor. But this layer is not a random collection of balls. Instead, they arrange themselves into a pattern.
During the MBE process, the empty ball pool is a vacuum chamber and the balls are high purity elements such as nitrogen and uranium that are heated until individual atoms can escape into the chamber.
The bottom of our imaginary ball pit is actually a charged underground that attracts the individual atoms. Atoms arrange themselves on the substrate to form a wafer of very thin material – in this case, uranium nitride.
Thin material sandwiches form heterostructures
Back in the ball pit, we created a layer of blue and red balls arranged in a pattern. Now let’s make another layer of green and orange balls on top of the first layer.
To study the quantum properties of these materials, Gofryk and his team will join two different material wafers into a sandwich called a heterostructure. For example, the thin layer of uranium nitride could be bonded to a thin layer of another material, such as gallium arsenide, a semiconductor. Interesting quantum mechanical properties can be observed at the junction between the two dissimilar materials.
“We can make sandwiches out of these materials from a variety of elements,” Gofryk said. “We have a lot of flexibility. We’re trying to think about the novel structures we can create with maybe some predicted quantum properties.”
“We want to look at electronic properties, structural properties, thermal properties and the transport of electrons through the layers,” he continued. “What happens if you lower the temperature and apply a magnetic field? Will it cause electrons to behave a certain way?”
INL is uniquely suited to actinide research
The INL is one of the few places where researchers can work with uranium and thorium for this type of science. The amounts of radioactive materials – and the resulting safety concerns – are comparable to the radioactivity found in an everyday smoke detector.
“The INL is the perfect place for this research because we are interested in this type of physics and chemistry,” said Gofryk.
In the end, Gofryk hopes the lab will lead to breakthroughs that will help attract the attention of potential collaborators and recruit new collaborators to the lab.
“These actinides have such special properties,” he said. “We hope that we can discover some new phenomena or new physics that have not been found before.”
Sidebar: What is Quantum Mechanics?
In 1900, the German physicist Max Planck first described how light emitted by heated objects, such as the filament of a light bulb, behaved like particles.
Since then, numerous scientists – including Albert Einstein and Niels Bohr – have explored and extended Planck’s discovery to develop the field of physics known as quantum mechanics. In short, quantum mechanics describes the behavior of atoms and subatomic particles.
Quantum mechanics differs from normal physics in part because subatomic particles have properties of both particles and waves at the same time, and their energy and motion occur in discrete quantities called “quanta”.
More than 120 years later, quantum mechanics plays a key role in numerous practical applications, particularly in lasers and transistors – a key component of modern electronic devices. Quantum mechanics also promises to serve as the basis for the next generation of computers, known as quantum computers, which will be much more powerful at solving certain types of calculations.
Why uranium and thorium are different
Uranium, thorium, and the other actinides have something in common that makes them interesting for quantum mechanics: the arrangement of their electrons.
Electrons do not orbit the nucleus like the earth orbits the sun. Rather, they dart around at random. But we can define areas where there is a high probability of finding electrons. These probability clouds are called “orbitals”.
For the smallest atoms, these orbitals are simple spheres surrounding the nucleus. However, as atoms get larger and contain more electrons, the orbitals begin to take on strange and complex shapes.
In very large atoms like uranium and thorium (92 and 90 electrons, respectively), the outermost orbitals are a complex collection of party balloon, jelly bean, dumbbell, and hula-hoop shapes. The electrons in these orbitals are highly energetic. While scientists can guess their quantum properties, no one knows exactly how they will behave in the real world.
Quantum Tunneling: When the Impossible Becomes Improbable
Quantum tunneling is an important part of a variety of phenomena, including nuclear fusion in stars, mutations in DNA, and diodes in electronic devices.
To understand quantum tunneling, imagine a toddler rolling a ball up a mountain. In this analogy, the ball is a particle. The mountain is a barrier, most likely a semiconductor material. In classical physics, the ball has no chance of having enough energy to fly over the mountain.
But at the quantum level, subatomic particles have properties of both particles and waves. The peak of the wave represents the highest probability of finding the particle. Thanks to a quirk of quantum mechanics, while most of the wave bounces off the barrier, if the barrier is thin enough, a small portion of that wave will travel through.
For a single particle, the small amplitude of this wave means that the probability that the particle will make it to the other side of the barrier is very small.
However, when a large number of waves propagate past a barrier, the probability increases, and sometimes a particle gets through. This is quantum tunneling.
Quantum Wells: Where Electrons Get Stuck
Quantum wells are also important, especially for devices like light emitting diodes (LEDs) and lasers.
Like quantum tunneling, building quantum wells requires alternating layers of very thin (10 nanometers) material, with one layer acting as a barrier.
While electrons normally move in three dimensions, quantum wells trap electrons in two dimensions within a barrier that is impossible to cross for practical purposes. These electrons exist at specific energies — such as the precise energies needed to produce specific wavelengths of light.
Team Script’s breakthrough quantum algorithm
Cody A. Dennett et al., Towards Actinide Heterostructure Synthesis and Science, nature communication (2022). DOI: 10.1038/s41467-022-29817-0
Provided by the Idaho National Laboratory
Citation: New Laboratory to Explore the Quantum Mysteries of Nuclear Materials (2022 October 18) Retrieved October 19, 2022 from https://phys.org/news/2022-10-laboratory-explore-quantum-mysteries-nuclear.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#laboratory #explore #quantum #mysteries #nuclear #materials

Leave a Comment