The American Society of Clinical Oncology (ASCO) and the American Association for Cancer Research (AACR) have issued updated guidance regarding the use of electronic nicotine delivery systems (ENDS). Roy S. Herbst, MD, Ph.D., is corresponding author of the statement released today in both Clinical Cancer Research and the Journal of Clinical Oncology.
The statement, which is an update of the version previously released in 2015, was developed by members of the Tobacco Products and Cancer Subcommittee of the AACR Committee on Science Policy and Governmental Affairs and the ASCO Subcommittee on Tobacco Cessation and Control of the Committee on Health Equity and Outcomes .
dr Herbst is a past chair of the Subcommittee on Tobacco Products and Cancer and current chair of the AACR Committee on Science Policy and Governmental Affairs. He is also Associate Director of Clinical Affairs and Chief of Medical Oncology at Yale Cancer Center and Smilow Cancer Hospital, Ensign Professor of Medicine (Medical Oncology) and Professor of Pharmacology, and Assistant Dean for Translational Research at Yale School of Medicine.
“Current evidence is lacking to support electronic nicotine delivery systems, particularly e-cigarettes, as a smoking cessation aid,” said Dr. Autumn. “However, their use has increased at an alarming rate and threatens to hamper progress against tobacco use. Another big problem is its use by youth and adults who have never used tobacco before.”
The new statement aims to detail advances in scientific understanding of the epidemic of electronic nicotine delivery systems, strengthen recommendations to protect public health, promote evidence-based tobacco cessation in all groups, and highlight areas where more research is needed.
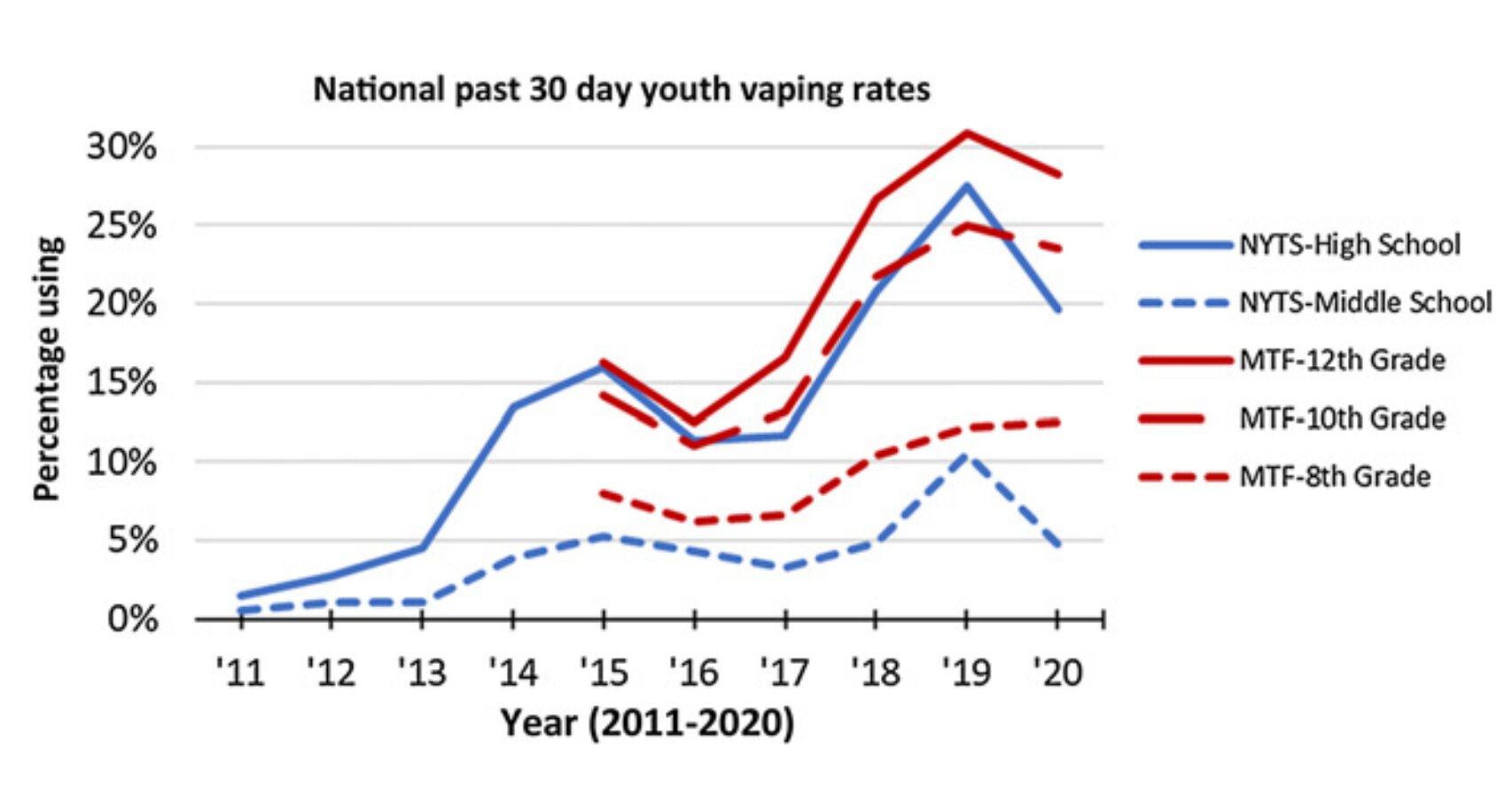
The first electronic nicotine delivery systems were introduced in the US in 2006 to deliver nicotine to users without burning tobacco. Some products can deliver a rapid release of a similar amount of nicotine as modern American cigarettes, contributing to a high potential for addiction.
“This is a call for urgent action by Congress, state legislatures and regulators to implement the various legal, regulatory and research recommendations outlined in this report, including the use of synthetic nicotine, with the goal of reducing its use by adolescents and Reducing adults who have never used tobacco before,” said Suchitra Krishnan-Sarin, Ph.D., Albert E. Kent Professor of Psychiatry at Yale. Dr. Krishnan-Sarin was recently appointed vice chair of the Subcommittee on Tobacco Products and Cancer .
Evidence-based treatments to promote smoking cessation and prevent smoking recurrence to reduce cancer incidence and improve public health remain a top priority for both ASCO and AACR. Together with Yale Cancer Center and Smilow Cancer Hospital, they recognize the urgent need for research to understand the relationship between electronic nicotine delivery systems and tobacco-related differences.
“Although electronic nicotine delivery systems emit fewer carcinogens than combustible tobacco, largely due to the lack of combustion products, it is clear that they still pose health risks,” said Dr. Autumn. “Use of these products exposes the user to carcinogens and therefore likely increases long-term risk of cancer, and e-cigarettes have hooked a new generation of adolescents and young adults to nicotine and threaten to impede progress against tobacco-related diseases.”
Claims of “tobacco-free nicotine” could lead non-smokers to try e-cigarettes
Roy S. Herbst et al., Electronic Nicotine Delivery Systems: An Updated Policy Statement from the American Association for Cancer Research and the American Society of Clinical Oncology, Clinical Cancer Research (2022). DOI: 10.1158/1078-0432.CCR-22-2429
Roy S. Herbst et al., Electronic Nicotine Delivery Systems: An Updated Policy Statement from the American Association for Cancer Research and the American Society of Clinical Oncology, Journal of Clinical Oncology (2022). DOI: 10.1200/JCO.22.01749
Provided by Yale Cancer Center
Citation: Updated Policy on Electronic Nicotine Delivery Systems (October 26, 2022), retrieved October 26, 2022 from https://medicalxpress.com/news/2022-10-policy-electronic-nicotine-delivery.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#Updated #Policy #Electronic #Nicotine #Delivery #Systems

Leave a Comment