Credit: Pixabay/CC0 Public Domain
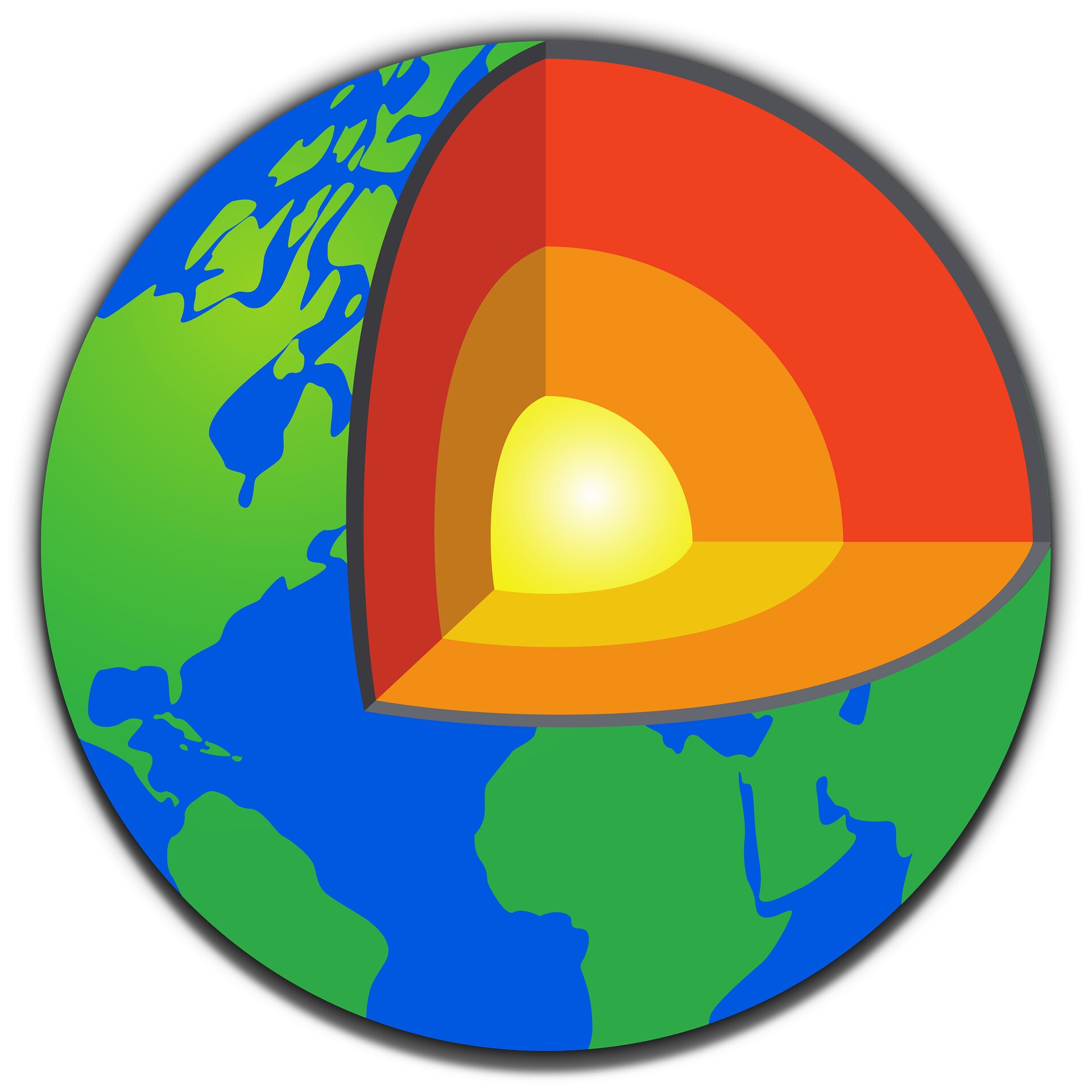
How is the earth structured? First of all, it consists of several layers: the crust, the upper and lower mantle and the core. The mantle makes up most of our planet’s volume – 84%. The lower mantle makes up 55% of Earth’s volume – it’s also hotter and denser than the upper mantle.
The lower mantle has played an important role in Earth’s evolution, including how the Earth cooled over billions of years, how materials were circulated, and how water is stored and transported from deep within over a geological timeframe.
For more than seven decades, the mineralogy of the lower mantle has been intensively studied. Decades of studies, including laboratory experiments, computer simulations, and the study of inclusions in deep diamonds, led to the conclusion that the lower mantle consists of three main minerals: bridgmanite, ferropericlase, and davemaoite.
In a recently published study in Nature, a team of scientists – including Byeongkwan Ko, former Ph.D. A student at ASU, now a postdoctoral fellow at Michigan State University, and Sang-Heon Dan Shim, professor at the School of Earth and Space Exploration and Navrotsky Professor of Materials Science at ASU, have performed a new high-pressure experiment using a few different styles of heating to reveal an additional mineral located in the lower mantle.
Among these three main minerals, two minerals, bridgmanite and davemaoite, both have so-called perovskite crystal structures. This structure is also well known in physics, chemistry, and materials engineering, since some materials with the perovskite-type structure have shown superconductivity.
At shallow depths, minerals with similar crystal structures often fuse and become individual minerals, typically in a high temperature environment. For example, mineral diopside contains both calcium and magnesium and is stable in the crust. However, despite the structural similarity, existing studies have shown that calcium-rich davemaoite and magnesium-rich bridgmanite remain separate throughout the lower mantle.
“Why don’t davemaoite and bridgmanite merge into one, even though they have very similar atomic structures? This question has intrigued researchers for over two decades,” Shim said. “Many attempts have been made to find conditions under which these two minerals fuse, but the experimental answer has consistently been two separate minerals. Here we felt that we needed some fresh new ideas in experiments.”
The new experiment was an opportunity for the research group to try different heating techniques to compare methods. Instead of slowly increasing the temperature in conventional high-pressure experiments, they increased the temperature very quickly to the high temperature associated with the lower mantle, reaching 3000–3500 F within a second. The reason for this was that as soon as two minerals with perovskite structure, it becomes very difficult for them to fuse together, even when exposed to temperature conditions where a single perovskite mineral should be stable.
By rapidly heating the samples to target temperatures, Ko and Shim avoided the formation of two perovskite-structure minerals at low temperatures. Once they reach lower mantle temperature, they use X-rays at the Advanced Photon Source to monitor mineral formation for 15–30 minutes. They found that only a single perovskite mineral forms, unexpected from the previous experiments. They found that at sufficiently high temperatures of more than 3500 F, davemaoite and bridgmanite become a single mineral in the perovskite-type structure.
“It was hypothesized that a large size difference between calcium and magnesium, the main cations of davemaoite and bridgmanite, respectively, should prevent these two minerals from merging,” Ko said. “But our study shows that they can overcome such differences in hot environments.”
The experiments suggest that the deeper, sufficiently high temperature lower mantle should have a different mineralogy than the shallower lower mantle. Because the mantle was much warmer in early Earth, the group’s new results suggest that most of the lower mantle then contained a single mineral with a perovskite structure, meaning the mineralogy differed from today’s lower mantle.
This new observation has a number of significant implications for our understanding of the depths of the Earth. Many seismic observations have shown that the properties of the deeper lower mantle differ from those of the shallower lower mantle. The changes are reported to be gradual. The merging of bridgmanite and davemaoite is shown to be gradual in the research group’s experiments.
In addition, the properties of a rock with three main minerals, bridgmanite, ferropericlase and davemaoite, do not match well with the properties of the deeper lower mantle. Ko and co-workers predict that these unresolved problems can be explained by a merger of bridgmanite and davemaoite into a new single mineral with a perovskite structure.
Quantum mechanical simulations of lower mantle minerals
Byeongkwan Ko et al, Calcium Dissolution in Deep Mantle Bridgmanite, Nature (2022). DOI: 10.1038/s41586-022-05237-4
Provided by Arizona State University
Citation: Researchers Discover Previously Unknown Deep Earth Mineralogy (2022, October 20) Retrieved October 20, 2022 from https://phys.org/news/2022-10-vorher-unknown-mineralogy-deep-earth.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#Researchers #discover #previously #unknown #mineralogy #deep #earth

Leave a Comment