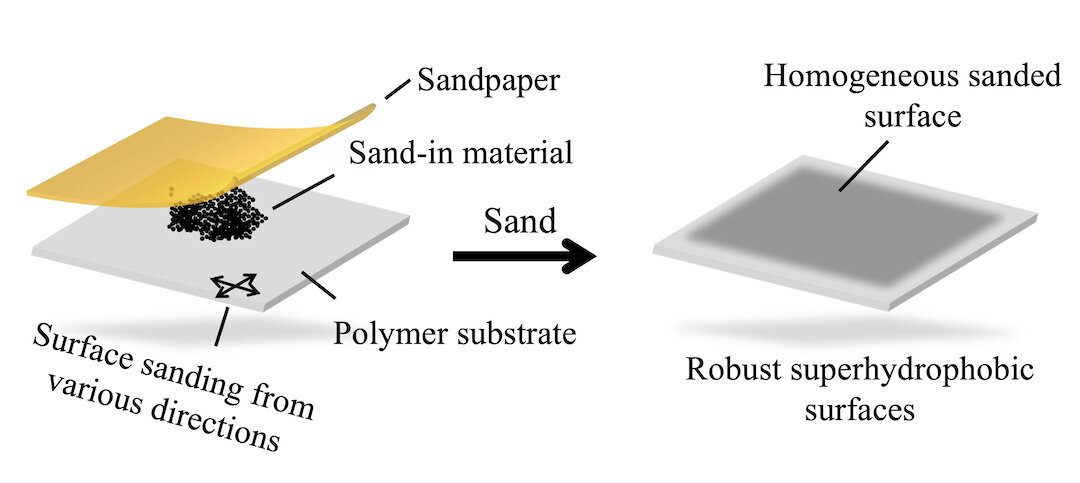
An illustration shows the one-sand technique developed by Rice to make materials superhydrophobic. The one-step sandpaper and powder process also gives the materials improved anti-icing properties. Photo credit: Weiyin Chen
Want a surface that won’t get wet? Take some sandpaper.
Researchers at Rice University have developed a simple way to make surfaces superhydrophobic—that is, highly water-repellent—without the chemicals often used in such processes.
Her technique involves sandpaper, an assortment of powders, and some elbow grease.
The laboratories of Rice Professors C. Fred Higgs III and James Tour, co-corresponding authors of an article in the American Chemical Society journal ACS Applied Materials and Interfaces, showed that sanding a surface increases its ability to shed water without getting wet. But grinding in a powder at the same time gives it hydrophobic superpowers.
Better still, their super hydrophobic surfaces also have excellent anti-icing properties. They found that water took 2.6 times longer to freeze on treated surfaces than on untreated materials. They also found that ice lost 40% of its adhesive strength even at temperatures as low as minus 31 degrees Fahrenheit.
How well a surface absorbs or repels water can be measured by analyzing the contact angle of droplets that settle there. To be superhydrophobic, a material must have a water contact angle—the angle at which the water surface meets the material surface—greater than 150 degrees. The larger the bead, the larger the angle. A zero degree angle is a puddle, while a maximum 180 degree angle is a sphere just touching the surface.
To achieve their super status, hydrophobic materials have low surface energy as well as a rough surface. The Rice team’s best materials showed a contact angle of about 164 degrees.
Higgs, whose lab specializes in tribology, the study of surfaces in sliding contact, said certain types of abrasive paper can provide a surface roughness that promotes the desired water-repellent, or hydrophobic, behavior.
“However, the Tour group’s idea of inserting selected powder materials between the friction surfaces during the sand-in process causes a tribofilm to form,” Higgs said. “This gives the additional advantage that the surface is functionalized in such a way that it repels water better and better.”
A tribofilm forms in a chemical reaction on surfaces sliding against one another. The surface of an engine piston is a good example, he said.
According to Higgs, grinding roughens up softer surfaces and allows the powder to stick by van der Waals forces. “These forces are greatest when surfaces come into close contact,” he said. “Therefore, powder particles can stick even after sanding in.”
Structural changes, as well as mass and electron transfer, appear to lower the surface energy of the materials, which the researchers say were already either mildly hydrophobic or hydrophilic prior to treatment.
The Rice team applied the technique to a variety of surfaces (Teflon, polyethylene, polypropylene, polystyrene, polyvinyl chloride, and polydimethylsiloxane) with a variety of powder additives. These included laser-induced graphene fibers, turbostratic flash graphene, molybdenum disulfide, Teflon, and boron nitride. Various aluminum oxide sandpapers ranging from 180 to 2000 grit were used.
The hard-wearing materials proved to be robust, because neither being heated to 130 degrees Celsius nor 18 months under the hot Houston sun bothered them. Sticking clear tape to the surface and peeling it 100 times didn’t degrade it either. But even as the materials began to fail, the labs found that re-grinding could easily refresh their hydrophobicity.
The team also discovered that materials can also be made hydrophilic or water-absorbent by changing sanding conditions and powder additives.
According to Tour, simplifying the manufacture of superhydrophobic and anti-icing materials should attract industry interest. “It’s difficult to make these materials,” he said. “Superhydrophobic surfaces do not allow water to collect. The water rolls off and rolls off, even at the smallest angle or light wind.
“Now almost any surface can be made superhydrophobic in seconds,” Tour said. “The powders can be as simple as Teflon or molybdenum disulfide, both of which are readily available, or newer graphene materials. Many industries could benefit, from aircraft and boat builders to skyscrapers where low-ice adhesion is essential. ”
“Airplane manufacturers don’t want ice forming on their wings, ship captains don’t want drag from seawater to slow them down, and biomedical devices need to avoid biofouling, which is the build-up of bacteria on wet surfaces,” Higgs said. “Tough, durable, superhydrophobic surfaces made with this one-step sand-in method can alleviate many of these problems.
“A limitation of other techniques for creating hydrophobic surfaces is that they cannot be scaled to large surface areas such as those found on airplanes and ships,” he said. “Simple application techniques like the one developed here should be scalable.”
Rice graduate student Weiyin Chen, co-lead author of the new paper, said the Tour lab has also applied its sand-in technique to various metal surfaces, including lithium and sodium foils for metal batteries, as reported in another recent paper.
“The spontaneous chemical reactions cause the formation of tribofilms, in this case the artificial solid electrolyte interphase,” Chen said. “The modified metals can be used as anodes for rechargeable metal batteries.”
Gas gives super properties to laser-induced graphene
Weiyin Chen et al, Robust Superhydrophobic Surfaces via the Sand-In Method, ACS Applied Materials and Interfaces (2022). DOI: 10.1021/acsami.2c05076
Provided by Rice University
Citation: Water can’t touch this sanded, powdered surface (4 August 2022), retrieved 4 August 2022 from https://phys.org/news/2022-08-sanded-powdered-surface.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#Water #touch #ground #powdered #surface

Leave a Comment