About the editor:
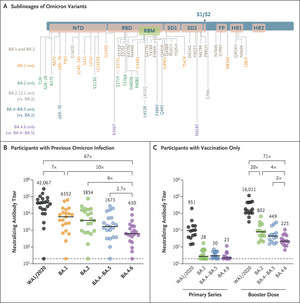
Neutralizing antibody responses to Omicron subvariants.
Panel A shows mutations in the SARS-CoV-2 spike protein in omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4–BA.5, and BA.4.6. BA.4 and BA.5 are grouped together because they have identical spike sequences. NTD denotes N-terminal domain, RBD receptor binding domain, RBM receptor binding motif, SD1 subdomain 1, SD2 subdomain 2, FP fusion peptide, HR1 heptad repeat 1 and HR2 heptad repeat 2; S1 and S2 indicate spike protein domains. Panel B shows neutralizing antibody titers by a luciferase-based pseudovirus neutralization assay in 19 participants recently infected with the BA.1 or BA.2 subvariant. All participants had been vaccinated except for the one participant who had a negative neutralizing antibody titer. Neutralizing antibody titers were measured against SARS-CoV-2 strain WA1/2020 and against Omicron subvariants BA.1, BA.2, BA.4–BA.5 and BA.4.6. Panel C shows neutralizing antibody titers in 16 participants 6 months after the first series of mRNA-1273 vaccination and after the mRNA-1273 booster dose. Neutralizing antibodies were measured against the SARS-CoV-2 strain WA1/2020 and against the omicron subvariants BA.2, BA.4–BA.5 and BA.4.6. In panels B and C mean neutralizing antibody titers are indicated by black horizontal bars and factor differences between strains are indicated at the top of the graph. The horizontal dashed lines indicate the lower limit of quantification.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant B.1.1.529 (Omicron) has fragmented into several subvariants with increased transmissibility and immune escape.1 At the time of this report, the omicron subvariant BA.5 is the dominant global virus and has demonstrated significant immune escape compared to previous omicron subvariants.2-5 BA.4.6 is a sublineage of BA.4 with two additional mutations in the spike protein (R346T and N658S) (Figure 1A) and has recently increased in prevalence in certain regions currently dominated by BA.5, including the United States. The ability of BA.4.6 to evade neutralizing antibodies induced by infection or vaccination remains to be determined.
We assessed neutralizing antibody titers against five SARS-CoV-2 strains – WA1/2020 and Omicron subvariants BA.1, BA.2, BA.4-BA.5 and BA.4.6 – in 19 participants recently infected with were subvariant omicron BA.1 or BA.2 and in 16 subjects vaccinated and boosted with the original mRNA-1273 vaccine (Moderna) (Table S1 in Appendix, available with the full text of this letter at NEJM.org ). ). In the cohort with prior omicron infection, all but one participant were vaccinated; Samples were collected a median of 21 days after diagnosis of Omicron infection. In this cohort, the mean pseudovirus-neutralizing antibody titers were 42,067 against WA1/2020, 6352 against BA.1, 3854 against BA.2, 1673 against BA.4–BA.5, and 630 against BA.4.6 (Figure 1B). The mean neutralizing antibody titers against BA.4.6 were 67-fold lower than the mean titers against WA1/2020, 10-fold against BA.1, 6-fold against BA.2 and against BA.4-BA.5 the factor 2.7.
In the mRNA-1273 vaccine cohort, participants were excluded if they had a known history of SARS-CoV-2 infection or positive results on a nucleocapsid serological analysis, or if they had received immunosuppressive drugs or other vaccines against SARS-CoV-2 . Six months after the first two mRNA-1273 immunizations, the mean neutralizing antibody titer was 951 against WA1/2020, 28 against BA.2, 30 against BA.4-BA.5, and 23 against BA.4.6 (Figure 1C). At a median of 17 days after the first booster dose, the median neutralizing antibody titers were 16,011 against WA1/2020, 802 against BA.2, 449 against BA.4-BA.5 and 225 against BA.4.6. The mean neutralizing antibody titer to BA.4.6 was 71-fold lower than that to WA1/2020, 4-fold to BA.2, and 2-fold to BA.4-BA.5.
Our data show that the BA.4.6 omicron subvariant significantly eluded neutralizing antibodies induced by infection or vaccination, with values 2 to 2.7 fold lower than the BA.5 titers, suggesting continued development of SARS-CoV-2. These results provide an immunological context for the increasing prevalence of BA.4.6 in populations where BA.5 is currently dominant. In addition, the R346T mutation has also recently been observed in other omicron subvariants, including BA.2.75 and BA.5, indicating the biological relevance of this mutation. The potential impact of BA.4.6 subvariant emergence on vaccine boosters containing BA.5 immunogens or on infection with BA.5 remains to be determined.
Nicole P. Hachmann, BS
Jessica Miller, B.S
Ai-ris Y. Collier, MD
Dan H. Barouch, MD, Ph.D.
Beth Israel Deaconess Medical Center, Boston, MA
[email protected]
Supported by a grant (CA260476) from the National Institutes of Health (NIH); through grants (to Dr. Barouch) from the Massachusetts Consortium for Pathogen Readiness, the Ragon Institute, and the Musk Foundation; and by a grant (AI69309, to Dr. Collier) from the NIH.
Disclosure forms provided by the authors with the full text of this letter are available at NEJM.org.
This letter was published on NEJM.org on October 19, 2022.
-
1. Baruch DH. Covid-19 vaccines – immunity, variants, boosters. N Engl. J Med 2022;387:1011–1020.
-
2. Hachmann NP, Mueller J, Necklace AY, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4 and BA.5. N Engl. J Med 2022;387:86–88.
-
3. Cao Y, Yisimayi A, jian f, et al. BA.2.12.1, BA.4 and BA.5 Escape antibodies elicited by Omicron infection. Nature 2022;608:593–602.
-
4. Khan K, Karim F, Ganga Y, et al. Omicron BA.4/BA.5 evades the neutralizing immunity evoked by BA.1 infection. Nat Commun 2022;13:4686–4686.
-
5. Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022;608:603–608.
#Neutralization #escape #SARSCoV2 #Omicron #subvariant #BA.4.6 #NEJM

Leave a Comment