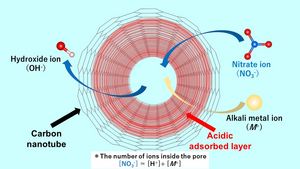
Image: An acid-adsorbed layer enhances the nano-encapsulation of nitrate anion impurities in single-walled carbon nanotubes (SWCNT) due to the strong confinement through the pore and the strong interaction between the layer and the anion. When the nitrate ions are adsorbed, the hydroxide ions are desorbed from the nanospace. Thus, the aqueous solution exhibits alkaline properties.
outlook more
Credit: Takahiro Ohkubo from the Okayama University Department of Chemistry, Graduate School of Natural Science and Technology, Okayama University
Efficient purification processes that separate contaminants from air and water are necessary to sustain life on earth. To this end, carbon materials have long been used to deodorize, separate and remove harmful anion impurities by adsorption. Until now, the detailed mechanism by which carbon purifies water has remained a mystery. In addition, it is not known whether the aqueous solution adsorbed on the carbon material is acidic, alkaline or neutral. To fill these gaps, researchers led by Dr. Takahiro Ohkubo, Associate Professor at the Department of Chemistry, Faculty of Natural Science and Technology, Okayama University, Japan, the basic mechanism by which anions are adsorbed by carbon nanopores.
In a recent article made available online on September 16, 2022 and published in Volume 629 Part B of the Journal of Colloid and Interface Sciences, the researchers report the use of Raman spectroscopic tools to study the adsorption of nitrate ions through the cylindrical pore of single-walled carbon nanotubes (SWCNTs). dr Ohkubo and his colleagues succeeded in deciphering the mechanism of acid layer formation near the pore walls. It turns out that when an aqueous solution containing ions penetrates into the carbon material, even if the aqueous solution is neutral, an acidic aqueous layer containing protons is formed, which maintains a stable state. dr Commenting on the novelty and fundamental nature of her work, Ohkubo said: “To date, there have been no reports showing the existence of acidic adsorption layers formed within nanotubes made of carbon materials.”
The research team, which also includes Dr. Nobuyuki Takeyasu, associate professor at the same faculty of Okayama University, found that the acidic layer facilitates efficient adsorption of the negatively charged nitrate anion impurities, with the adsorbed amount of nitrate ions being much larger than the cations or the positively charged groups. In addition, hydroxide ions are generated as counter ions. The anions present in the bulk solution are exchanged with the hydroxide ions in the SWCNT, making the aqueous solution alkaline. The team studied anion adsorption using several alkali metal nitrates, including lithium nitrate, sodium nitrate, rubidium nitrate and cesium nitrate solutions. They found that more nitrate ions are adsorbed than metal ions. The amount of proton adsorption was almost the same regardless of the type of alkali metal ion used. dr Ohkubo notes: “The acidic layer in the pore can strongly adsorb the nitrate anion species due to both the strong confinement by the pore and the strong interaction between the layer and the anion.”
Indeed, the findings are important steps towards the design and development of carbon nanotubes suitable for ion adsorption and purification of water and air. The purification mechanism presented in this research is a novel model that explains the alkalinity of the aqueous dissolution medium, which until now has been a mystery. The researchers note that the results of their study strongly suggest the need to neutralize water before use when ionic impurities are trapped by carbon materials. Another notable contribution of this study is the demonstration that the interface of nanomaterials is a novel chemical reaction field that could guide further experiments. Taken together, this work brings our understanding of the mechanism of anion adsorption by carbon to the next level and paves the way for novel carbon nanotubes as efficient purifiers.
About Okayama University, Japan
As one of the leading universities in Japan, Okayama University aims to create and establish a new paradigm for the sustainable development of the world. Okayama University offers a wide range of academic fields that become the basis of the integrated graduate schools. This not only allows us to conduct the most advanced and up-to-date research, but also provides an enriching educational experience.
Website: https://www.okayama-u.ac.jp/index_e.html
About Associate Professor Takahiro Ohkubo from Okayama University, Japan
dr Takahiro Ohkubo is Associate Professor in the Department of Chemistry, Graduate School of Natural Science and Technology, Okayama University. He is actively involved in research on topics such as structure and properties of nanoconfined systems, nanostructures (clusters and small particles), porous materials, surface science, and carbon-based materials. dr Ohkubo has received several awards throughout his academic career, including the Best Contribution Award in Education, Faculty of Science, Okayama University (2017) and the 2012 Young Researcher Award from the Colloid and Interface Chemistry Division of the Chemical Society of Japan (2012). “. . He has authored over 100 articles that have contributed significantly to his field of research.
diary
Journal of Colloid and Interface Sciences
research method
Experimental study
subject of research
Not applicable
article title
Acid layer-enhanced nano-encapsulation of anions in a cylindrical pore of a single-walled carbon nanotube
Article publication date
September 16, 2022
COI statement
The authors declare no conflicts of interest.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of the press releases published on EurekAlert! by contributing institutions or for the use of information about the EurekAlert system.
#acidic #layer #singlewalled #carbon #nanotubes #facilitates #entrapment #anion #impurities

Leave a Comment