WATERLOO – When that pesky cold hits and you’re stuck in bed with a runny nose sneezing, wouldn’t it be nice to have an inhaler that could kill the virus in its tracks?
Or what about more severe viruses like SARS-CoV-2 or even cancers like glioblastoma?
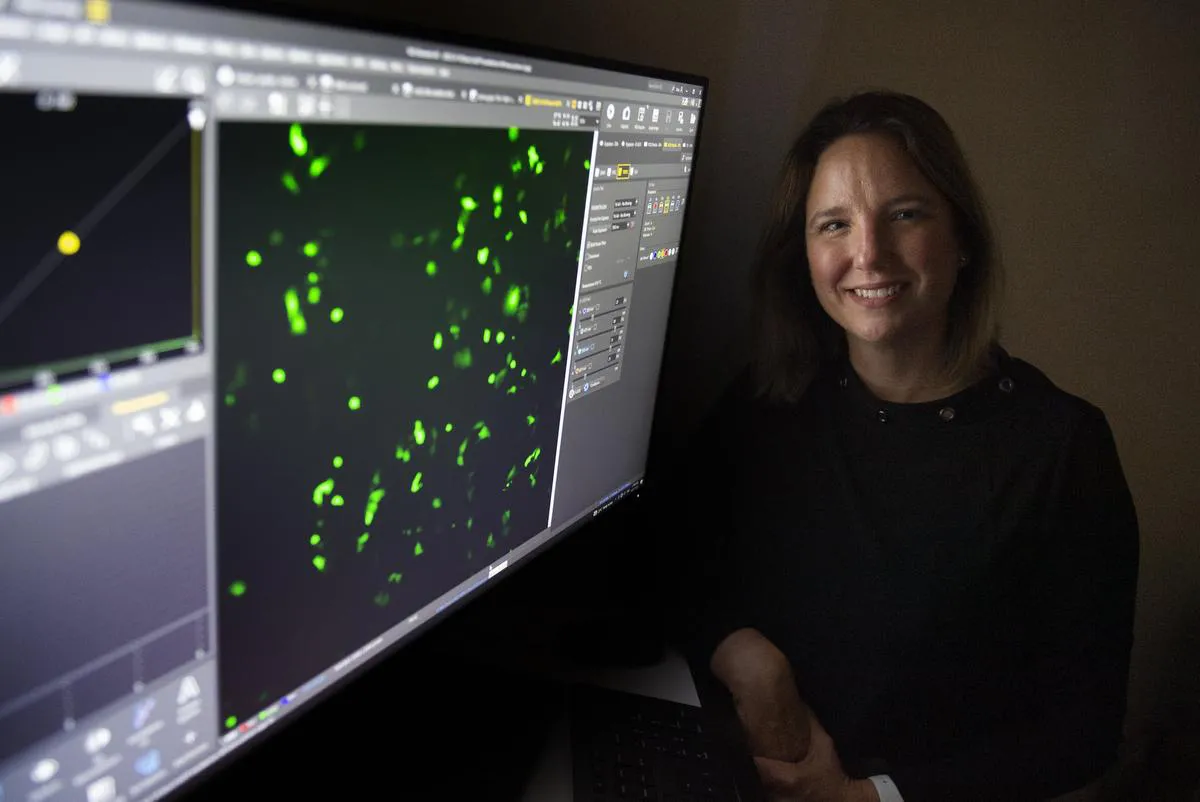
A new discovery by Wilfrid Laurier University virologist and immunologist Stephanie DeWitte-Orr has the potential to change the way we target and kill cancer and viral genes in the human body.
“Sometimes cells make proteins that they’re not supposed to make, or they make too much of a protein and it makes you sick,” said DeWitte-Orr, who was recently named Laurier’s 2022-23 University Research Professor. “What we found is a way to specifically turn off these proteins.”
The research was recently featured in an article published in the journal “Frontiers in Immunology” focuses on long double-stranded ribonucleic acid, or dsRNA, a nucleic acid produced by viruses. In the body, a healthy cell uses dsRNA as a “red flag” to recognize when it has been infected with a virus and to trigger an immune response.
In plants and invertebrates, researchers had previously been able to use dsRNA to target and stop the spread of disease in healthy and cancerous cells.
So far, however, this has never been possible in humans.
The process, known as RNA interference, allows DeWitte-Orr to insert a variety of different sequences into the same molecule that can target a variety of different viruses or cancer cells in the body.
“So we can use these big, long molecules to insert a lot of sequences to turn off a lot of different proteins at once,” she said.
She gives the example of a person who has a cancer cell that produces oncogenes that turn cells into tumor cells.
“If we can engineer our molecule to target these oncogenes and knock them out, we can stop the cancer cell,” DeWitte-Orr said.
In the lab, her team was able to successfully demonstrate this method on human lung cells infected with SARS-COV-2, the virus that causes COVID-19.
The method has also been successful with other coronaviruses that cause the common cold, and it has also worked on human brain cancer cells.
“For viruses, we’ve seen up to a 90 percent drop in virus production,” she said. “And with cancer, we can actually kill the cancer cell in a day or two.”
In previous research in this area, scientists injected too much dsRNA into a test subject to target the cells, which does not replicate a natural response to a virus. Using a smaller amount of the dsRNA better mimics the response to a real virus, DeWitte-Orr said, and allows for a more controlled response to the virus.
“We could put in all the influenza sequences for the flu variants that are circulating today, and then we could also throw in some coronavirus variants and put it in a molecule, you could breathe it in, and your airways would be protected against infection,” she said.
Their team is already attracting interest from a few outside companies who are interested in working with them on the project.
“It’s such a paradigm shift, and paradigm shifts in science are always difficult to accept at first,” she said. “We are trained as scientists to be critical thinkers, but I think we now have enough data behind us to say, ‘Yes, there is a phenomenon here.’ ”
She just submitted a grant to try the dsRNA process in preclinical studies in living subjects. So far it has only been successful in laboratory dishes. The next step requires moving to more complex systems, which will include preclinical studies in a hamster model with SARS-CoV-2. If successful, it will move on to human trials, a process that may take years.
At the tissue level, she also begins testing for glioblastoma, a rare type of brain tumor.
“Any situation where a cell is doing too much of something, you could use our technology in that application,” she said. “This technology has incredible potential, but we can only move forward as fast as resources support it. So we need money, because we can only do the research that the money funds.”
#Lauriers #discovery #change #target #kill #virus #cancer #genes

Leave a Comment