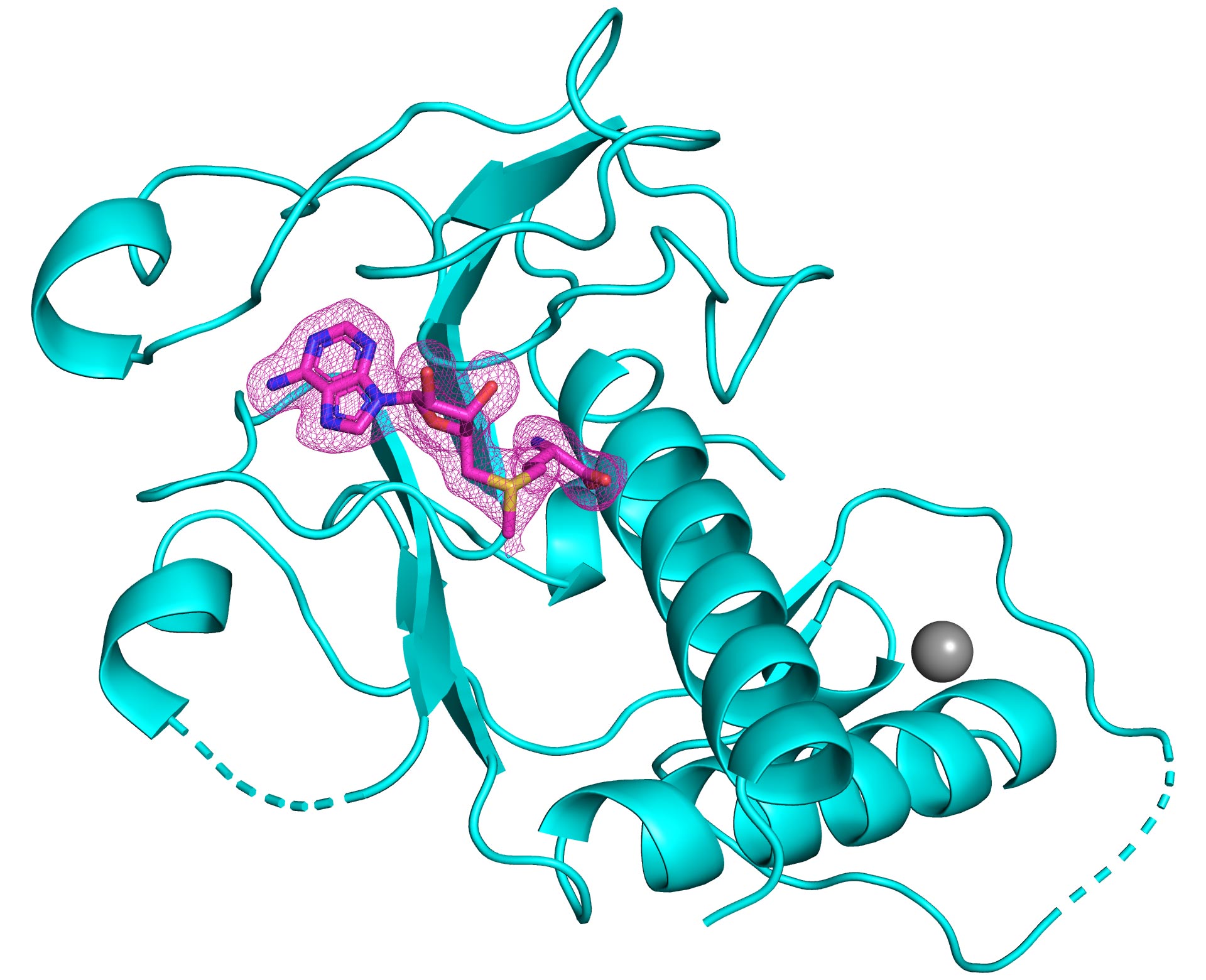
3-D structure of the nsp14 methyltransferase domain of SARS-CoV-2 (shown in cyan) bound to its natural cofactor S-adenosylmethionine (shown in pink mesh). Photo credits: Kottur, et al.; Nature Structural and Molecular Biology
Researchers unravel the crystal structure of a key enzyme from
” data-gt-translate-attributes=”[{” attribute=””>SARS-CoV-2, paving the way for new antivirals.
A high-resolution crystal structure of an enzyme essential to the survival of SARS-CoV-2, the virus that causes
The enzyme, known as nsp14, contains a critically important region known as the
“Part of what drives our work,” says Dr. Aggarwal, “is the knowledge gained from treating HIV—that you typically need a cocktail of inhibitors for maximum impact against the virus.”
The Mount Sinai research team actually developed three crystal structures of nsp14, each with different cofactors. From these, they identified the best scaffold for the design of antivirals for inhibiting the RNA methyltransferase activity that the enzyme enables and the virus needs to survive. According to their scheme, the antiviral would take the place of the natural cofactor S-adenosylmethionine, therefore preventing the methyltransferase chemistry from occurring. The crystal structures that the researchers have elucidated have been made available to the public. They can now serve as guides for biochemists and virologists globally to engineer these compounds.
Making the discovery possible was the ability of scientists to clear a hurdle that had prevented others in the past from creating three-dimensional crystals of the nsp14 methytransferase domain. “We employed an approach known as fusion-assisted crystallization,” explains lead author Jithesh Kottur, PhD. He is a postdoctoral fellow at Icahn Mount Sinai, and a crystallographer and biochemist. “It involves fusing the enzyme with another small protein that helps it to crystalize.”
Dr. Aggarwal is an internationally recognized structural biologist. He underscores the importance of ongoing investigative work by researchers in his field against a virus that has led to millions of deaths globally. “The virus evolves so quickly that it can develop resistance to the antivirals now available, which is why we need to continue developing new ones,” he observes. “Because of the high sequence conservation of nsp14 across coronaviruses and their variants (meaning it does not mutate much), our study will aid in the design of broad-spectrum antivirals for both present and future coronavirus outbreaks.”
Reference: “High resolution structures of the SARS-CoV-2 N7-methyltransferase inform therapeutic development” 8 September 2022, Nature Structural & Molecular Biology.
DOI: 10.1038/s41594-022-00828-1
Funding: NIH/National Institutes of Health, DOE/US Department of Energy, NIH/National Institute of General Medical Sciences, DOE/US Department of Energy
#Crystal #structure #key #SARSCoV2 #enzyme #decoded #paving #antiviral #COVID #agents

Leave a Comment