In a recent article in the magazine PLOS PATHOGENscientists summarized acute and persistent clinical manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The review also provides an overview of the efficacy and usefulness of preclinical animal models to understand the mechanistic details of acute and prolonged coronavirus disease 2019 (COVID-19).
Review: After the virus is gone – Can preclinical models be used for long-COVID research? Credit: Dmitry Demidovich/Shutterstock
Acute and long episodes of COVID
SARS-CoV-2, the causative agent of COVID-19, is a respiratory virus that belongs to the human betacoronavirus family. The virus primarily affects airway epithelial cells and causes a variety of symptoms, including fever, cough, fatigue, headache, shortness of breath, loss of taste and smell, neurological complications and, rarely, blood clotting.
Although mostly asymptomatic or mildly symptomatic, acute SARS-CoV-2 infection can lead to severe respiratory complications, multi-organ failure and even death in susceptible individuals.
COVID-19 symptoms do not always resolve with the resolution of an acute primary infection. About 30-75% of COVID-19 patients continue to suffer from specific symptoms even after clinical recovery. This condition is known as “Long COVID”. The most common long-term COVID symptoms are fatigue, dyspnea, arthralgia, myalgia, cardiac complications, and memory problems.
According to the National Institute for Health and Care Excellence (NICE), UK, long-term COVID symptoms can last 4 to 12 weeks, or even longer in some cases. Therefore, it has been hypothesized that persistent inflammation, viral persistence, and virus-induced autoimmune responses may be responsible for long-term manifestations of COVID-19.
As with acute COVID-19, long COVID symptoms are more common in the elderly and those with comorbidities. Pro-inflammatory cytokines have been shown to be predictive of long COVID in studies examining potential biomarkers during recovery from acute infections. In addition, elevated blood levels of urea, D-dimer, and C-reactive protein, and decreased lymphocyte levels are associated with long-term COVID symptoms.
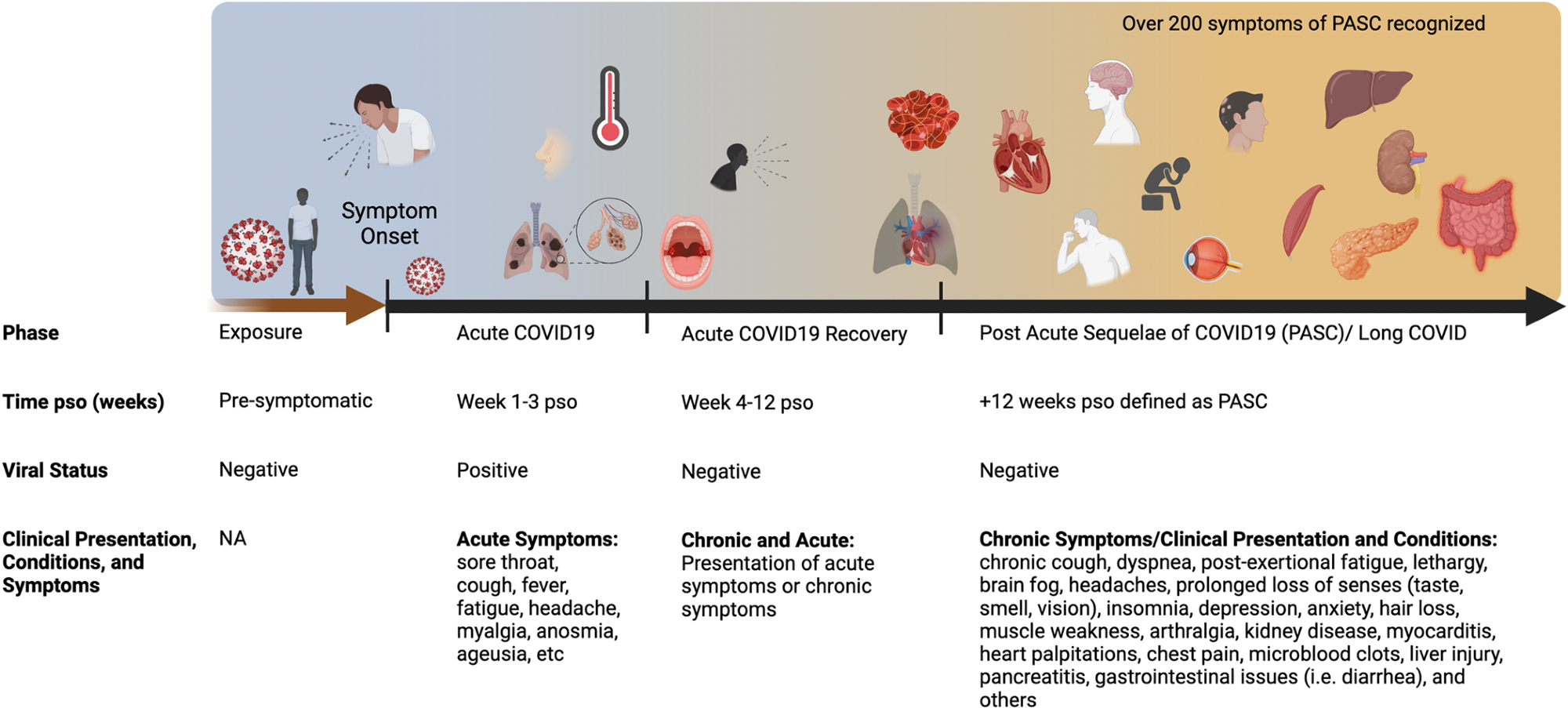 Acute COVID-19 includes symptoms that last up to 4 weeks. Live virus is typically present in the respiratory tract for 1 week pso, although it varies from person to person. Common symptoms of acute COVID-19 are summarized, including pulmonary and systemic manifestations. Long COVID or PASC includes persistent symptoms that are present 4 weeks after acute infection. The diverse multi-organ complications of PASC are summarized. COVID-19, coronavirus disease 2019; PASC, post-acute consequences of COVID-19; pso, occurrence after onset of symptoms.
Acute COVID-19 includes symptoms that last up to 4 weeks. Live virus is typically present in the respiratory tract for 1 week pso, although it varies from person to person. Common symptoms of acute COVID-19 are summarized, including pulmonary and systemic manifestations. Long COVID or PASC includes persistent symptoms that are present 4 weeks after acute infection. The diverse multi-organ complications of PASC are summarized. COVID-19, coronavirus disease 2019; PASC, post-acute consequences of COVID-19; pso, occurrence after onset of symptoms.
COVID-19 animal models to study the long-term consequences of COVID-19
Several animal models, including mice, hamsters, ferrets, and non-human primates, have been used throughout the pandemic to study SARS-CoV-2 transmission and infection dynamics, as well as therapeutic interventions.
respiratory complications
Studies examining respiratory complications associated with long COVID in hamsters have demonstrated persistence of the immune response even after systemic removal of SARS-CoV-2. Persistent infiltration of immune cells into the lungs and the presence of pneumonia and lung lesions have been observed in non-human primates after clinical recovery from COVID-19.
Since it is known that common long COVID symptoms are associated with a sustained immune response, these animal models could be used to study the etiology of prolonged airway complications.
On the other hand, ferret models are not considered suitable for long-term COVID studies as they develop only mild upper respiratory tract infection of SARS-CoV-2.
In humanized mice expressing human immune cells and human angiotensin-converting enzyme 2 (ACE2), sustained innate immune and inflammatory responses, persistent presence of viral RNA, weight loss and pulmonary fibrosis were noted 28 days after SARS-CoV-2 infection . Thus, humanized mice could serve as a valuable model for long-term COVID studies.
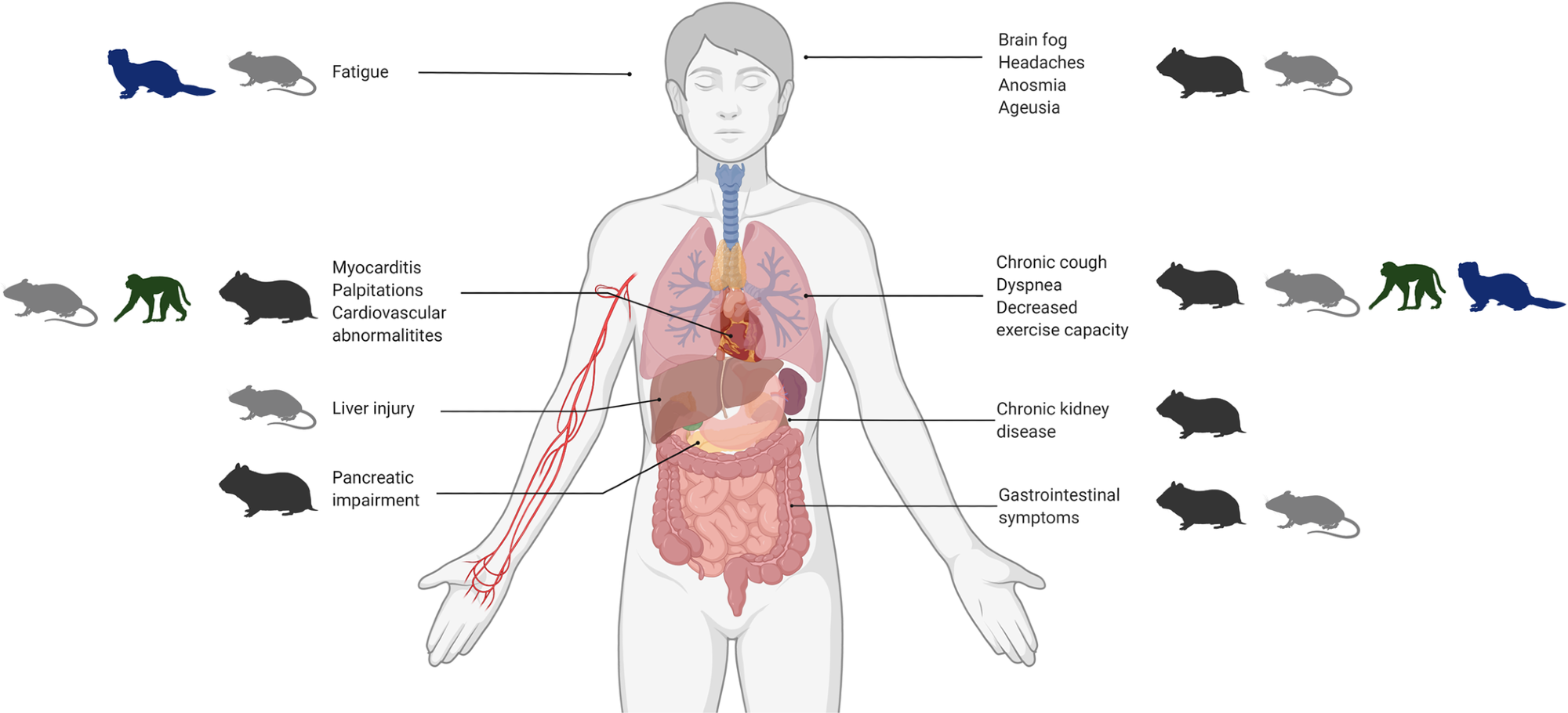 The four main groups of animal models (hamsters, mice, ferrets and NHPs) are outlined with corresponding human manifestations of PASC. COVID-19, coronavirus disease 2019; NHP, non-human primate; PASC, Post-acute consequences of COVID-19.
The four main groups of animal models (hamsters, mice, ferrets and NHPs) are outlined with corresponding human manifestations of PASC. COVID-19, coronavirus disease 2019; NHP, non-human primate; PASC, Post-acute consequences of COVID-19.
Multiorganic and systemic complications
Animal models that could be used to study multiorgan complications of long-term COVID include human ACE2-expressing mice, ferrets, and hamsters. In these animals, the presence of viral RNA was observed in several non-respiratory organs. Progressive bone loss was observed in hamsters 60 days after infection.
Non-human primates have been found to exhibit clinical manifestations and systemic immune responses similar to those of COVID-19 patients during the post-acute phase of infection. Therefore, these animals could be used to study damage caused by the immune system that has been observed in long-term COVID.
cardiovascular complications
Palpitations, chest pain and myocarditis are common symptoms of long COVID, indicating cardiovascular involvement. Hamsters could serve as a valuable model to study cardiac complications of long-term COVID-19, as they exhibit cardiovascular damage (thickening of the ventricular wall, increased ventricular mass-to-body mass ratio, and interstitial coronary fibrosis) up to 14 days after infection.
Humanized mice and non-human primates could also be used to study cardiac complications as viral RNA was detected in their cardiac tissues 5-6 weeks post-infection.
Neurological Complications
Neuropsychiatric complications, including olfactory dysfunction, headaches, cognitive decline, depression and anxiety, have been observed in patients with long-term COVID-19 illness.
Studies examining the olfactory dysfunction in SARS-CoV-2-infected hamsters have shown that the virus causes severe damage to the olfactory epithelium during the acute phase of infection. However, regular recovery of olfactory function was observed soon after viral clearance.
Regarding long-term effects, persistent impairment of cognitive and behavioral activities was observed in hamsters after SARS-CoV-2 infection. Taken together, these observations underscore the importance of hamster models for studying the long-term neurological outcomes of COVID-19.
Therapeutic interventions in long-COVID
Multiple clinical trials are underway to identify potential therapeutic interventions for long COVID. Most of these studies examine treatment options for respiratory complications, with some focusing on extra-respiratory manifestations.
A phase 2 clinical trial in patients with long-term COVID-19 is evaluating the therapeutic efficacy of ribonuclease RSLV-132, an RNase-Fc fusion protein that prevents aberrant activation of the innate immune system. Since the persistent presence of subgenomic viral RNA has been observed in non-human primates, they can serve as an important model system to study the therapeutic efficacy of ribonuclease RSLV-132.
Sodium pyruvate, which has been found to reduce long COVID symptoms in patients, could be tested in hamsters for detailed therapeutic characterization as it shows similarities to humans in long COVID multiorgan symptoms.
Taken together, these observations underscore the importance of different animal models in exploring the pathogenesis and therapeutic interventions of long COVID.
#preclinical #COVID19 #models #unravel #mystery #long #COVID


Leave a Comment