An implanted fruit fly Drosophila melanogaster (foreground) interacts with an intact fruit fly (background). Photo credit: Alain Herzog (EPFL)
EPFL scientists have developed an implantation technique that allows unprecedented optical access to the ‘spinal cord’ of the fruit fly Drosophila melanogaster. This work can potentially lead to breakthroughs in neuroscience, artificial intelligence, and bio-inspired robotics.
Understanding biological motor control requires the ability to record neuronal activity as animals behave,” says Professor Pavan Ramdya from EPFL’s School of Life Sciences. “We have a billion neurons in the human spinal cord — a huge system — and we can’t manipulate neurons in a human the way we can in animals. Drosophila, the fruit fly, is a very small organism in which one can genetically manipulate and map the activity of almost the entire motor circuit in behaving animals.”
For years, Ramdya’s research has focused on digitally recapitulating the principles underlying Drosophila motor control. In 2019, his group released DeepFly3D, a deep learning-based motion capture software that uses multiple camera views to quantify the 3D limb movements of flies. In 2021, Ramdya’s team presented LiftPose3D, a method for reconstructing 3D animal poses from 2D images captured by a single camera. This effort was complemented by his publication in 2022 of NeuroMechFly, a first morphologically accurate digital “twin” of Drosophila.
But it is precisely in this area, which lies at the interface between biology, neuroscience, computer science and robotics, that new challenges are always waiting. The aim is not only to map and understand the nervous system of an organism – an ambitious task in itself – but also to find out how to develop bio-inspired robots that are as agile as flies.
“The obstacle we faced prior to this work,” says Ramdya, “was that we could only record motor circuits from flies for a short time before the animal’s health deteriorated.”
For this reason, Ramdya teamed up with Professor Selman Sakar from EPFL’s School of Engineering to develop tools to monitor Drosophila neuronal activity over longer periods of time, up to and including the insect’s entire lifespan. This project was led by Laura Hermans, a Ph.D. Student co-supervised by Ramdya and Sakar.
A window into the ventral nerve cord
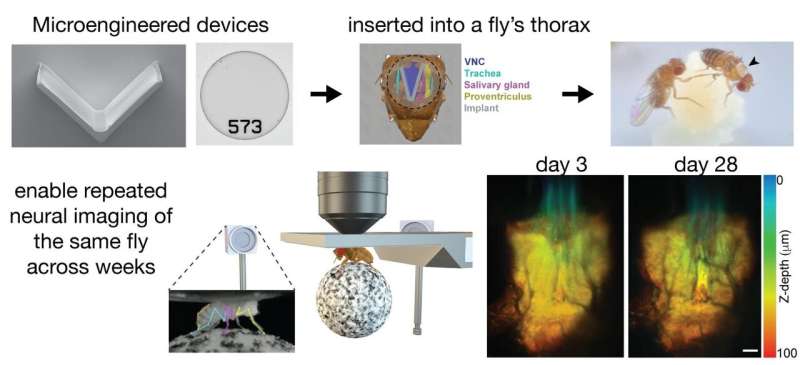
“We have developed microengineered devices that provide optical access to the animal’s ventral nerve cord,” says Herman, referring to the fly’s equivalent of the spinal cord. “We then surgically implanted these devices in the fly’s thorax,” she continues. “One of these devices, an implant, allows us to move the fly’s organs sideways to expose the ventral nerve cord underneath. Then we seal the thorax with a transparent micro-machined window. Once we have flies with these devices, we can record the fly’s behavior and its neuronal activity in a variety of experiments over long periods of time.
The purpose of all these tools is to allow scientists to observe a single animal over long periods of time. You can now conduct experiments that extend beyond a few hours and can even cover the entire lifespan of the fly. “We can, for example, investigate how an animal’s biology adapts during the course of the disease,” says Hermans. “We can also study changes in the activity and structure of neural circuits during aging. The ventral nerve cord of the fly is ideal because it houses the animal’s motor circuits, allowing us to study how locomotion evolves over time or after injury.”
The implant
“As engineers, we crave such well-defined technical challenges,” says Selman Sakar. “Pavan’s group developed a dissection technique to remove from the fly the organs that block the field of view and visualize the ventral nerve cord. However, the flies can only survive a few hours after the operation. We were convinced that an implant has to be placed in the thorax. There are analogous techniques for visualizing the nervous system of larger animals such as rats. We were inspired by these solutions and started thinking about miniaturization.”
The early prototypes attempted to meet the challenge of safely moving and holding the fly’s organs aside to expose the ventral nerve cord while allowing the fly to survive post-surgery.
The surgical procedure in which the new V-shaped implant is inserted into the thorax of the fruit fly to allow optical access to its ventral nerve cord. Photo credit: Laura Hermans (EPFL)
“For this challenge you need someone who can approach a problem from both a life sciences and engineering perspective – this underscores the importance of Laura [Hermans] and Murats [Kaynak] work,” says Sakar.
The early implants were rigid and very few flies survived the procedure. Trying to improve survival rates without sacrificing image quality presented a challenge that required multiple design iterations. In the end, the winner was a simple but effective prototype: a V-shaped, compliant implant that can safely deflect the fly’s organs aside, exposing the abdominal cord and allowing researchers to close the hole in the cuticle with a “thorax with barcode “window” that allows them to observe the ventral nerve cord and measure neural activity while the fly goes about its daily life.
“Given the different anatomy from animal to animal, we had to find a safe and adaptable solution,” says Sakar. “Our implant addresses this special need. Together with the development of appropriate tissue micromanipulation tools and a 3D nanoprinted compatible stage for mounting animals during repeated imaging sessions, we offer a complete, versatile toolkit for neuroscience research.”
An open road
The achievement is an example of the open and interdisciplinary research typical of EPFL. “From day one we were very open to sharing the technology,” says Sakar. “The idea here is to spread the tools and methods quickly so that we can facilitate both the advancement of the technology and the discovery process that it offers in many areas of research. A number of groups, I think, want to explore our technology.”
“By studying the fly, we believe that understanding something relatively simple can lay the groundwork for understanding more complicated organisms,” says Ramdya. “When you learn mathematics, you don’t dive into linear algebra; you first learn how to add and subtract. Also, it would be fantastic for robotics to understand how even a “simple” insect works.”
The next step for the team is to use their new methodology to unravel the mechanisms of Drosophila movement control. “Biological systems are really unique compared to artificial systems because they can dynamically modulate, for example, the excitability of neurons or the strength of synapses,” adds Ramdya. “In order to understand what makes biological systems so agile, you have to be able to observe these dynamics. In our case, for example, we want to study how motor systems respond to aging or during recovery from injury over the course of an animal’s life.”
The current study was published in nature communication.
New technology reveals limb control in flies – and maybe in robots
Laura Hermans et al, Microengineered devices enable long-term imaging of the ventral nerve cord in behaving adult Drosophila, nature communication (2022). DOI: 10.1038/s41467-022-32571-y
Provided by the Ecole Polytechnique Federale de Lausanne
Citation: A window into the nervous system of the fruit fly (2022, September 13), retrieved September 13, 2022 from https://phys.org/news/2022-09-window-fruit-nervous.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#window #nervous #system #fruit #fly


Leave a Comment