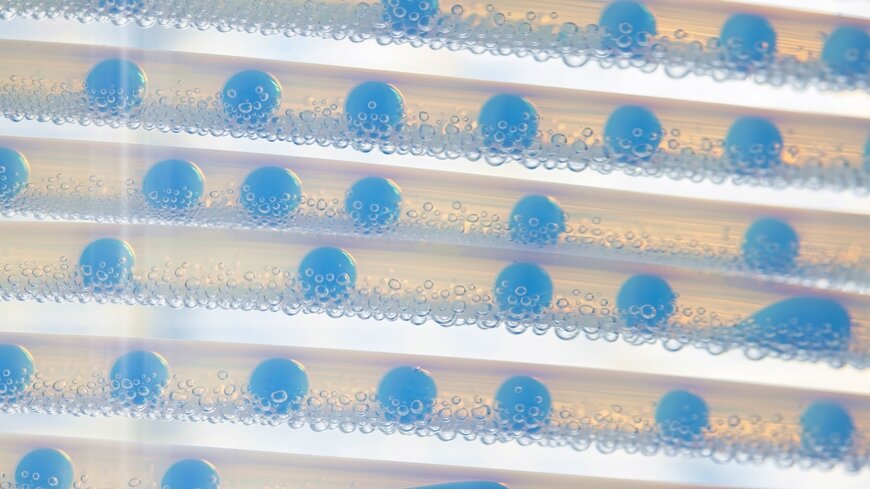
Conveyor belt: A different chemical mixture is created in each of the droplets within the “tubular flow reactor” – under exactly the same boundary conditions. Photo credit: Empa
Nature strives for chaos. That’s a nice, comforting sentence when another coffee cup has been tipped over the computer keyboard and you imagine that you can wish the sugar-sweet, milky brew back into the coffee cup – right where it was seconds before. But wishing won’t work. Because as I said, nature strives for chaos.
Scientists have coined the term entropy for this effect – a measure of disorder. If the disruption increases, processes run spontaneously in most cases and the way back to the previously prevailing order is blocked. See the spilled coffee cup. Even combined heat and power plants that generate a huge cloud of steam from a neat pile of wood or a pile of hard coal over their cooling tower work entropy-driven. In many combustion processes, the disorder increases dramatically – and people take advantage of this by tapping a bit of energy from the ongoing process in the form of electricity for their own purposes.
Can entropy stabilize something?
Crystals are seen as the very opposite of disorder. In a crystal structure, all the elements of the lattice are neatly sorted close together in the smallest of spaces. The idea that crystals could be stabilized by the power of entropy and thus create a new class of materials is all the more bizarre.
Entropy-stabilized materials are still a young field of research. It started in 2004 with so-called high-entropy alloys, mixtures of five or more elements that can be mixed together. If the mixture is successful and all elements are distributed homogeneously, special properties sometimes arise that do not come from the individual ingredients, but from their mixture. Scientists call this the “cocktail effect”.
Chaos reigns even in the heat
It has been known since 2015 that ceramic crystals can also be stabilized by the “power of disorder”. In this way, oversized and tiny elements that would normally destroy it also fit into the crystal. The Empa research team has already succeeded in incorporating nine different atoms into a crystal. The advantage is that they remain stable even at high temperatures – because a “rearrangement” would lead to more order. The natural striving for maximum disorder thus stabilizes the unusual crystal structure – and thus the entire material.
“With up to four components in the crystal everything is still normal, from five components the world changes,” explains Michael Stuer, researcher in Empa’s Advanced Ceramics department. Since the researcher, who grew up in Luxembourg, came to Empa in 2019, he has been working in the research field of high-entropy crystals. “This material class opens up many new possibilities for us,” says Stuer. “We can stabilize crystals that would otherwise disintegrate due to internal stresses. And we can create highly active crystal surfaces that have never been seen before and look for interesting cocktail effects.”
Together with his colleague Amy Knorpp, Stuer is now heading into the unknown. The two are specialists in the production of fine crystal powder and have colleagues at Empa for X-ray and surface analysis to precisely characterize the samples they produce. With their help, Michael Stuer now wants to be at the top of the international scene. “The number of publications on the subject of high-entropy crystals is currently increasing rapidly. And we want to be there right from the start,” says the researcher.
islands of knowledge
What is required now is a systematic approach, specialist knowledge and a good deal of perseverance. where do you start Which direction do you take? “Currently there is no coherent expertise, no overall overview of this new research area,” says Stuer. “Various research groups around the world are working on limited projects. This creates individual islands of knowledge that will have to grow together over the next few years.”
Michel Stuer and Amy Knorpp focus on catalytically active materials. The chemical reaction you are interested in involves the formation of CO2 and hydrogen to methane. The aim is to turn a greenhouse gas into a sustainable, storable fuel. “We know that CO2 Molecules adsorb particularly well on certain surfaces and the desired reaction then proceeds more easily and quickly,” says Amy Knorpp. “Now we are trying to produce entropic crystals with such highly active regions on their surfaces.”
chemical assembly line
In order to make faster progress, the researchers built a special synthesis device with the help of the Empa workshop, in which many different chemical mixtures can be tested one after the other, like on an assembly line. In the “Segmented Flow Tubular Reactor”, small bubbles run through a tube in which the respective reaction takes place. At the end, the bubbles are emptied and the powder contained can be further processed.
“The ‘Tubular Flow Reactor’ has a great advantage for us: all the bubbles are the same size, which is why we always have ideal and constant boundary conditions for our syntheses,” explains Stuer. “If we need larger quantities of a particularly promising compound, we simply produce several bubbles with the same compound one after the other.”
The windows on the right
The precursor powder is then transformed into fine crystals of the desired size and shape through various drying processes. “Crystals are like houses, they have closed outer walls and some have windows,” explains Michael Stuer. Sometimes the shape of the crystal already indicates the window side. For example, when a mixture forms needle-shaped crystals. “The long sides of the needle are the lower-energy ones. Not much happens there. The crystal edges at the tips of the needles, on the other hand, are highly energetic. That’s where it gets interesting,” says Stuer.
For their first major project, the Empa researchers have teamed up with colleagues from the Paul Scherrer Institute (PSI). They are investigating the possible methanation of CO2 from biogas plants and sewage treatment plants in an experimental reactor. The PSI researchers have already gained experience with various catalysts and repeatedly come up against a problem: the catalyst on the surface of which the chemical reaction takes place weakens over time. This is because sulfur components in the biogas contaminate the surface or the catalyst surfaces undergo chemical transformation at high temperatures.
Here the researchers are looking for a breakthrough with entropic crystals; after all, these do not disintegrate even at high temperatures – they are stabilized by chaos. “We hope that in doing so, our crystals will last longer and possibly be more resistant to sulfur pollution,” says Stuer.
Drawing a map
After that, the crystal specialists at Empa are ready for other challenges such as high-performance batteries, superconducting ceramics or catalysts for car exhaust fumes and other chemical production processes. “It’s a dark forest we’re going into,” says Amy Knorpp. “But we have a guess as to which direction something might be found. Now let’s draw a map of these systems.
Her latest research results are published in CHIMIA.
The theory predicts a new type of bonding that assembles nanoparticle crystals
From Synthesis to Microstructure: Development of the High Entropy Ceramic Materials of the Future, CHIMIA (2022). DOI: 10.2533/chimia.2022.212
Provided by the Federal Materials Testing and Research Institute
Citation: In control of chaos to engineer high-entropy ceramics (9 August 2022), retrieved 10 August 2022 from https://phys.org/news/2022-08-chaos-high-entropy-ceramics.html
This document is protected by copyright. Except for fair trade for the purpose of private study or research, no part may be reproduced without written permission. The content is for informational purposes only.
#Rule #Chaos #evolve #High #Entropy #Ceramics

Leave a Comment