Despite widespread vaccination adoption, COVID-19 remains a serious public health concern for high-risk patients due to the emergence of new variants of concern (VOCs), dwindling immunity, and breakthrough infections.
At a recent symposium held in Singapore, esteemed experts Dr. Asok Kurup, Consultant Infectious Disease Physician at Mount Elizabeth Medical Centre, Singapore, and Dr. Alex Soriano, Head of the Department of Infectious Diseases, Barcelona Hospital Clinic and Assistant Professor, University of Barcelona, Spain, provided insights into evolving global trends since the start of the COVID-19 pandemic and discussed key challenges in treating high-risk patients with COVID- 19 in intensive care. They also highlighted early treatment strategies to minimize the burden of COVID-19 on healthcare systems.
Identify people at risk for serious illness
The majority of Singapore’s population has been vaccinated and refreshed, but there are still vulnerable groups who have suboptimal responses to vaccination, such as the elderly with frequent comorbidities and the immunocompromised.
“In the new normal, surveillance for new VOCs should be maintained as future variants could be more virulent and infect more people,” Kurup warned. “There is an urgent need to support frontline healthcare workers to avoid exhaustion from COVID-19 combat fatigue. Vulnerable people should be isolated at home or in care facilities. To minimize hospital and intensive care admissions, it is important to identify those at risk for serious illnesses early so that interventions are calibrated according to risks.”
Risk factors for severe COVID-19
Risk factors for severe COVID-19 in adults can be classified into host factors, societal factors, and viral factors. Host factors include older age, male gender, obesity, and underlying conditions such as pulmonary disease, cardiovascular disease (CVD), immunosuppression, and chronic kidney disease. [Lancet 2020;395:497-506;
Lancet 2020;395:1054-1062; JAMA Intern Med 2020;180:934-943] Stacked comorbidities increase risk of severe COVID disease (illustration 1).
“In certain parts of the world, societal and structural factors such as poverty and racism may put marginalized groups at greater risk. Viral factors such as inoculum size and variant also affect risk,” added Kurup.
Dealing with COVID-19 in the intensive care unit
Dealing with COVID-19 in the ICU comes with many challenges. Critically ill adults with COVID-19 are at increased risk for thromboinflammatory diseases. Endothelial damage and hypercoagulability play critical roles in the progression of severe COVID-19, with endothelial dysfunction being an important cause of organ failure. [N Eng J Med 2020;383:120-128;
CMAJ 2020;192(40):E1156-E1161; Intensive Care Med 2021;47:86-89]
The treatment is complex. In contrast to the typical acute respiratory distress syndrome (ARDS), COVID-19 pneumonia has distinct phenotypes (type H vs. L) that require individualized therapy and selection of appropriate emergency treatment. [Crit Care 2020:24:154;
JAMA 2020;323:2329-2330; Eur J Anaesthesiol 2022;39:445-451]
Early treatment slows the progression of the infection
The clinical spectrum of COVID-19 infection is broad, ranging from completely asymptomatic to progressive, life-threatening viral pneumonia. “In the Omicron era, approximately 85-95 percent of cases are asymptomatic to mild, while 5-10 percent of cases are moderate to severe,” Soriano said.
The rapid viral replication seen in respiratory viruses drives rapid progression to severe infection. The increase in viral load triggers an inflammatory response to try to control viral replication. “The window for viral load reduction is generally within the first 5 days after viral replication begins,” Soriano shared. “In the majority of the vaccinated population, virus replication lasts 5-10 days and triggers an inflammatory immune response sufficient to fight the infection. However, patients predisposed to developing severe infection (patients with older age, comorbidities, and gene mutations or autoantibodies) often have dysregulated immune responses; these are associated with longer viral replication times of >10 days, leading to a higher risk of progression to pneumonia, ARDS, and thrombosis (figure 2). [Clin Infect Dis 2021;72:1467-1474]
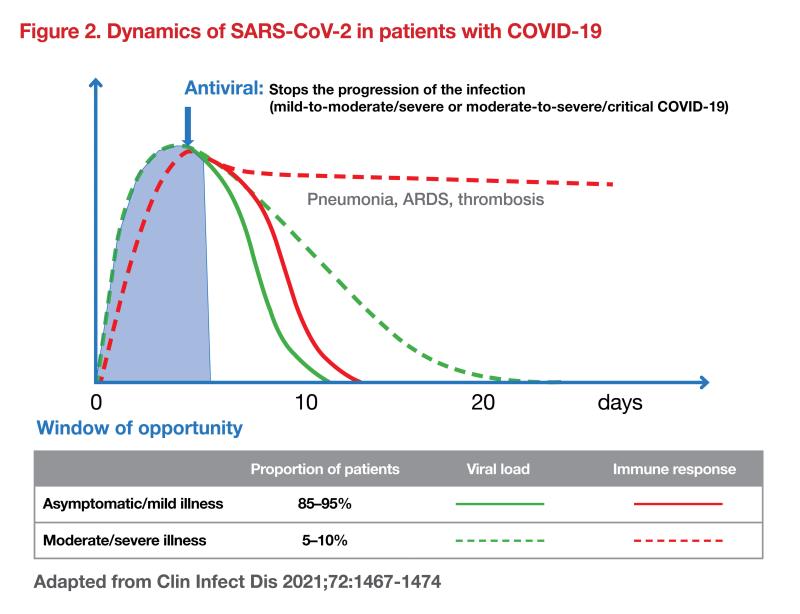
“In these high-risk groups, it is important to assess the influence of antivirals on the course of the disease and hospital admission,” emphasized Soriano. “Early treatment with antivirals slows or stops the progression of infection from mild to moderate to severe or from moderate to severe to critical COVID-19.”
Nirmatrelvir/ritonavir for mild to moderate COVID-19
A cohort study demonstrated the importance of timely antiviral treatment in COVID-19 patients. Three groups of adult hospitalized patients with mild to moderate COVID-19 who were at high risk of progression to severe disease were analyzed; one group was treated with nirmatrelvir/ritonavir within the first 5 days after onset of symptoms (treatment ≤ 5 days), another group received nirmatrelvir/ritonavir 5 days after onset of symptoms (treatment > 5 days), while a third group was untreated became antivirals (untreated). After adjusting for severity variables, nirmatrelvir/ritonavir use was associated with a significantly shorter time to negative RT-PCR conversion (10 days vs. 17 days) in patients treated ≤5 days after symptom onset and in untreated patients, respectively . However, no difference was observed between the “untreated” and “treated >5 days” cohorts. [Clin Infect Dis 2022;ciac600]
In the Phase II/III Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) study in symptomatic, unvaccinated, non-hospitalized adults at high risk of progression to severe COVID-19, nirmatrelvir reduced /ritonavir significantly reduced the risk of hospitalization for COVID-19 or death from any cause by 87.8 percent (0.77 percent vs. 6.31 percent for placebo; p<0.001) among those who started treatment within 5 days of starting treatment symptoms started. [N Engl J Med 2022;386:1397-1408]
“While the results of the subgroup analyzes were consistent across subgroups, there was a trend suggesting a greater benefit of nirmatrelvir/ritonavir versus placebo in the subgroup of patients with older age (≥ 65 years vs. < 65 years), higher BMI ( ≥ 30kg/m2 versus 25-30 kg/m2 versus <25 kg/m2 ), higher viral loads versus lower viral loads, and a higher number of comorbidities at baseline (≥2 versus <2),” Soriano said.
Another study of nirmatrelvir/ritonavir during the Omicron era, using real-world data from the Israel Clalit Health Services (CHS) database and the Israel Ministry of Health (MOH) COVID-19 database, showed that combination therapy with was associated with a significant increase in the rate of severe COVID-19 or mortality (hazard ratio [HR]0.54, 95 percent confidence interval [CI], 0.39-0.75). It appeared to be more effective in elderly, immunocompromised patients and patients with underlying neurological or cardiovascular disease (interaction p<0.05 for all). [Clin Infect Dis 2022;ciac443] Of 180,351 eligible patients, 2.6 percent received nirmatrelvir/ritonavir and 75.1 percent had an adequate COVID-19 immunization status.

#Timely #treatment #patients #risk #severe #COVID19 #prevents #consequences #Latest #news #doctors #nurses #pharmacists #respirology

Leave a Comment